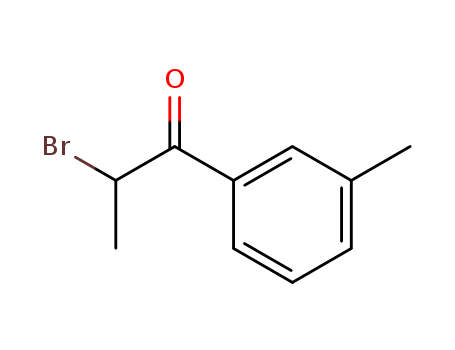
2-bromo-3-methylpropiophenone 1451-83-8
- CasNo:1451-83-8
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
1451-83-8 Properties
- Molecular Formula:C10H11 Br O
- Molecular Weight:227.101
- Boiling Point:134-135 °C(Press: 8 Torr)
- PSA:17.07000
- Density:1.357±0.06 g/cm3(Predicted)
- LogP:2.96110
1451-83-8 Usage
Application
2-bromo-3-methylpropiophenone employed as an important intermediate for raw material for organic synthesis, agrochemical, pharmaceutical and dyestuff field. Also used as intermediate for 4-methylmethcathinone.
Agricultural Uses
2-bromo-3-methylpropiophenone is a useful compound in the research.
1451-83-8 Relevant articles
Novel benzene-based carbamates for ache/bche inhibition: Synthesis and ligand/structure-oriented sar study
Bak, Andrzej,Kozik, Violetta,Kozakiewicz, Dariusz,Gajcy, Kamila,Strub, Daniel Jan,Swietlicka, Aleksandra,Stepankova, Sarka,Imramovsky, Ales,Polanski, Jaroslaw,Smolinski, Adam,Jampilek, Josef
, (2019/05/10)
A series of new benzene-based derivatives was designed, synthesized and comprehensively characterized. All of the tested compounds were evaluated for their in vitro ability to potentially inhibit the acetyl-and butyrylcholinesterase enzymes. The selectivity index of individual molecules to cholinesterases was also determined. Generally, the inhibitory potency was stronger against butyryl-compared to acetylcholinesterase; however, some of the compounds showed a promising inhibition of both enzymes. In fact, two compounds (23, benzyl ethyl(1-oxo-1-phenylpropan-2-yl)carbamate and 28, benzyl (1-(3-chlorophenyl)-1-oxopropan-2-yl) (methyl)carbamate) had a very high selectivity index, while the second one (28) reached the lowest inhibitory concentration IC50 value, which corresponds quite well with galanthamine. Moreover, comparative receptor-independent and receptor-dependent structure–activity studies were conducted to explain the observed variations in inhibiting the potential of the investigated carbamate series. The principal objective of the ligand-based study was to comparatively analyze the molecular surface to gain insight into the electronic and/or steric factors that govern the ability to inhibit enzyme activities. The spatial distribution of potentially important steric and electrostatic factors was determined using the probability-guided pharmacophore mapping procedure, which is based on the iterative variable elimination method. Additionally, planar and spatial maps of the host–target interactions were created for all of the active compounds and compared with the drug molecules using the docking methodology.
Annulation Cascades of 2-Bromo-1-arylpropan-1-ones with Terminal Alkynes Involving C-Br/C-H Functionalization
Ouyang, Xuan-Hui,Hu, Chao,Song, Ren-Jie,Li, Jin-Heng
supporting information, p. 4659 - 4662 (2018/08/09)
Straightforward access to various substituted naphthalenones by copper-catalyzed [4 + 2] annulation cascades of 2-bromo-1-arylpropan-1-ones with terminal alkynes is presented. Employing a Cu(MeCN)4PF4 catalyst and 1,10-phenanthroline (1,10-Phen) ligand enables the formation of three new C-C bonds in a single reaction via [4 + 2] annulation of a 2-bromo-1-arylpropan-1-one with an alkyne followed by α-alkylation with the other 2-bromo-1-arylpropan-1-one with excellent functional group tolerance and step efficiency.
CERAMIDE GALACTOSYLTRANSFERASE INHIBITORS FOR THE TREATMENT OF DISEASE
-
Paragraph 000388; 000389; 000431; 000432, (2018/01/17)
Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments containing such compounds, and methods of using such compounds to treat or prevent diseases or disorders associated with the enzyme ceramide galactosyltransferase (CGT), such as, for example, lysosomal storage diseases. Examples of lysosomal storage diseases include, for example, Krabbe disease and Metachromatic Leukodystrophy.
Hybrid dopamine uptake blocker-serotonin releaser ligands: A new twist on transporter-focused therapeutics
Blough, Bruce E.,Landavazo, Antonio,Partilla, John S.,Baumann, Michael H.,Decker, Ann M.,Page, Kevin M.,Rothman, Richard B.
supporting information, p. 623 - 627 (2014/07/07)
As part of our program to study neurotransmitter releasers, we report herein a class of hybrid dopamine reuptake inhibitors that display serotonin releasing activity. Hybrid compounds are interesting since they increase the design potential of transporter related compounds and hence represent a novel and unexplored strategy for therapeutic drug discovery. A series of N-alkylpropiophenones was synthesized and assessed for uptake inhibition and release activity using rat brain synaptosomes. Substitution on the aromatic ring yielded compounds that maintained hybrid activity, with the two disubstituted analogues (PAL-787 and PAL-820) having the most potent hybrid activity.
1451-83-8 Process route
-
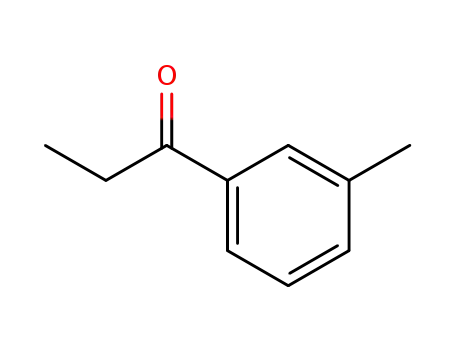
-
51772-30-6
3'-Methylpropiophenone

-
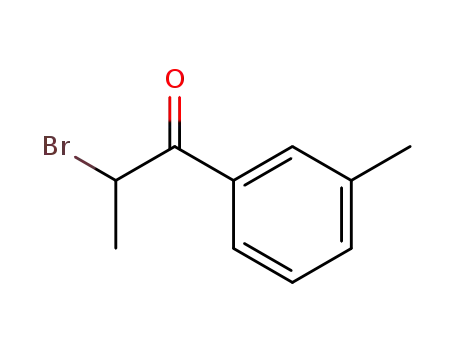
-
1451-83-8
2-bromo-1-(3-methylphenyl)propan-1-one
| Conditions | Yield |
|---|---|
|
With
bromine;
In
dichloromethane;
at 20 ℃;
for 0.5h;
|
100% |
|
With
bromine;
In
dichloromethane;
for 10h;
Inert atmosphere;
|
59% |
|
3'-Methylpropiophenone;
With
bromine;
In
dichloromethane;
for 10h;
Inert atmosphere;
In
dichloromethane;
|
59% |
|
With
bromine;
In
diethyl ether;
|
|
|
With
bromine; acetic acid;
|
|
|
With
bromine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide; toluene-4-sulfonic acid;
In
acetonitrile;
Reflux;
|
|
|
With
bromine;
In
dichloromethane;
|
-
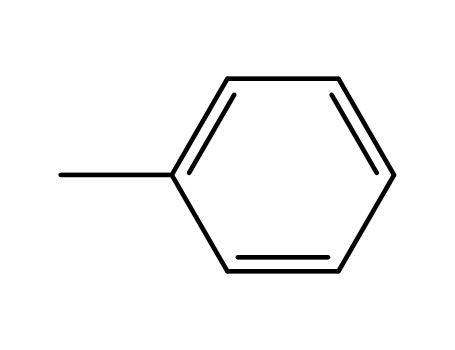
-
108-88-3,15644-74-3,16713-13-6
toluene

-

-
1451-83-8
2-bromo-1-(3-methylphenyl)propan-1-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: aluminum (III) chloride / dichloromethane
2: bromine / dichloromethane
With
aluminum (III) chloride; bromine;
In
dichloromethane;
1: |Friedel-Crafts Acylation;
|
1451-83-8 Upstream products
-
51772-30-6

3'-Methylpropiophenone
-
108-88-3

toluene
1451-83-8 Downstream products
-
1193779-18-8
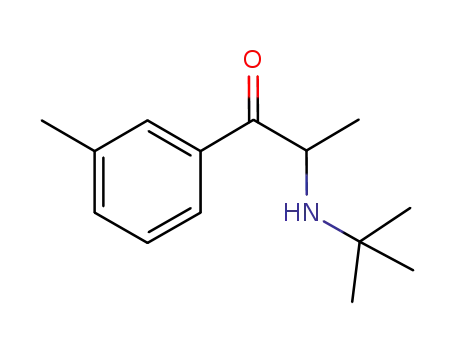
2-(N-tert-butylamino)-3'-methylpropiophenone
-
1350768-23-8
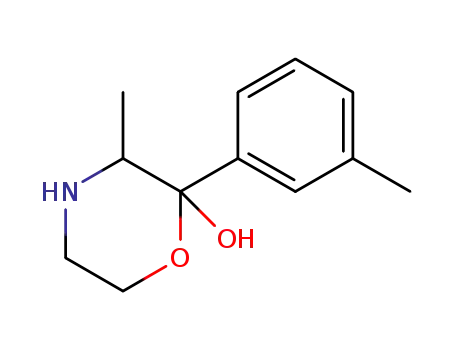
PAL-589
-
1214939-96-4
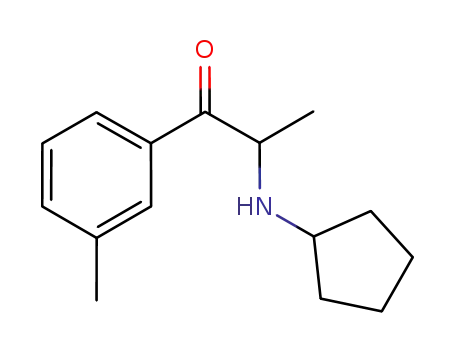
2-(N-cyclopentylamino)-3'-methylpropiophenone
-
1214940-01-8
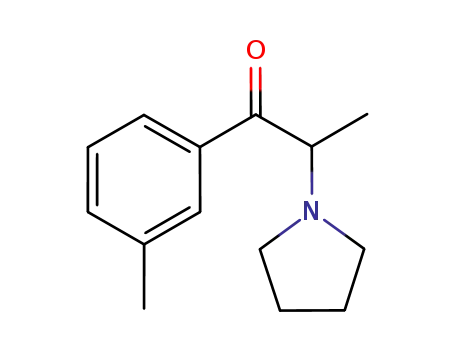
2-(N-pyrrolidinyl)-3'-methylpropiophenone








