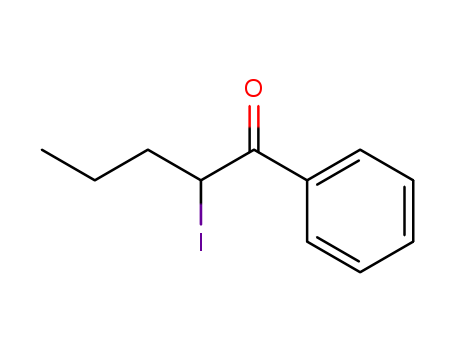
2-iodo-1-phenyl-pentane-1-one 124878-55-3
- CasNo:124878-55-3
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
124878-55-3 Properties
- Molecular Formula:C11H13IO
- Molecular Weight:288.128
124878-55-3 Relevant articles
Metal catalyst-free direct α-iodination of ketones with molecular iodine
Rao, Maddali L.N.,Jadhav, Deepak N.
, p. 6883 - 6886 (2007/10/03)
Ketones are directly converted to the corresponding α-iodoketones in good yields with molecular iodine under metal catalyst-free conditions. A significant difference in the reactivities was observed for aliphatic and aromatic ketones; whereas aliphatic ke
CHEMISTRY OF α-NITROEPOXIDES: SYNTHESIS OF USEFUL INTERMEDIATES VIA NUCLEOPHILIC RING OPENING OF α-NITROEPOXIDES
Vankar, Yashwant D.,Shah, Kavita,Bawa, Anita,Singh, Surendra P.
, p. 8883 - 8906 (2007/10/02)
Various α-nitroepoxides are converted into corresponding 1,2-diketones via two different ways of ring opening viz. with Pd(O) and with DMSO/BF3*EtO2 (or ClSiMe3).In addition to this, a variety of nucleophiles are reacted with α-nitrocyclopentene oxide 6 and α-nitrocyclohexene oxide 7 to form the corresponding α-substituted ketones which are useful intermediates in organic synthesis.Two of the products so obtained viz. 32 and 33 are also transformed further into optically active thialactones 38 and 39 respectively via baker's yeast reduction followed by lactonisation.
A Facile Conversion of α-Nitroepoxides into 1,2-Diones and α-Iodoketones
Vankar, Yashwant D.,Saksena, Rajendra K.,Bawa, Anita
, p. 1241 - 1244 (2007/10/02)
1,2-Diones and α-iodoketones have been prepared from α-nitroepoxides by treatment with DMSO/BF3*Et2O followed by Et3N and BF3*Et2O/NaI respectively.
124878-55-3 Process route
-
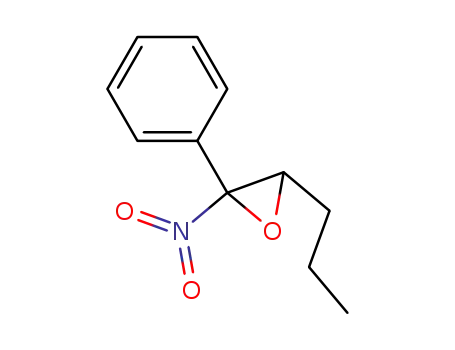
-
124878-57-5
3-propyl-2-nitro-2-phenyl oxirane

-
-
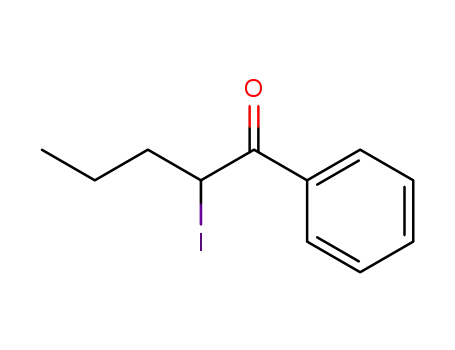
124878-55-3
2-iodo-1-phenyl-pentane-1-one
| Conditions | Yield |
|---|---|
|
With
boron trifluoride diethyl etherate; sodium iodide;
In
acetonitrile;
at 0 ℃;
for 8h;
|
68% |
|
With
boron trifluoride diethyl etherate; sodium iodide;
In
acetonitrile;
at 0 ℃;
for 8h;
|
68% |
-
-
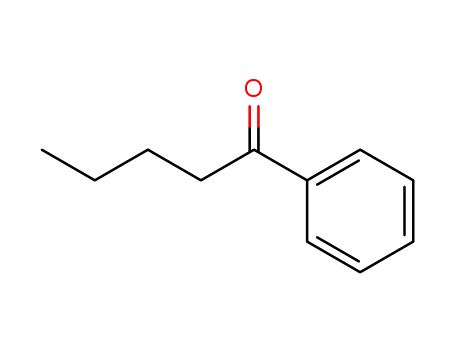
1009-14-9
phenyl butyl ketone

-

-
124878-55-3
2-iodo-1-phenyl-pentane-1-one
| Conditions | Yield |
|---|---|
|
With
iodine;
In
1,2-dimethoxyethane;
at 90 ℃;
for 3h;
|
87% |
124878-55-3 Upstream products
-
124878-57-5

3-propyl-2-nitro-2-phenyl oxirane
-
1009-14-9

phenyl butyl ketone