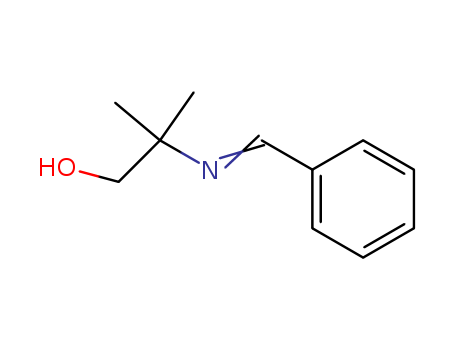
2-(benzylideneamino)-2-methylpropan-1-ol 22563-90-2
- CasNo:22563-90-2
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
22563-90-2 Properties
- Molecular Formula:C11H15NO
- Molecular Weight:177.246
- Vapor Pressure:0.00146mmHg at 25°C
- Boiling Point:283.8°C at 760 mmHg
- Flash Point:167.8°C
- Density:0.94g/cm3
22563-90-2 Relevant articles
Synthesis and antibacterial activity of aromatic and heteroaromatic amino alcohols
de Almeida, Camila G.,Reis, Samira G.,de Almeida, Angelina M.,Diniz, Claudio G.,da Silva, Vania L.,Le Hyaric, Mireille
experimental part, p. 876 - 880 (2012/06/18)
Two series of aromatic and heteroaromatic amino alcohols were synthesized from alcohols and aldehydes and evaluated for their antibacterial activities. All the octylated compounds displayed a better activity against the four bacteria tested when evaluated by the agar diffusion method and were selected for the evaluation of minimal inhibitory concentration. The best results were obtained for p-octyloxybenzyl derivatives against Staphylococcus epidermidis (minimal inhibitory concentrations = 32μm).
AMIDOPYRAZOLE DERIVATIVE
-
Page/Page column 83-84, (2010/11/23)
A platelet coagulation inhibitor which inhibits neither COX-1 nor COX-2 is provided. The inhibitor is a compound represented by general formula (I): wherein Ar1 and Ar2 independently represent a 5- or 6-membered aromatic heterocyclic group optionally substituted with 1 to 3 substituents, or a phenyl group optionally substituted with 1 to 3 substituents; R1 represents a lower acyl group, carboxyl group, a lower alkoxycarbonyl group, a lower alkoxy group, a lower alkyl group optionally substituted with 1 or 2 substituents, a carbamoyl group optionally substituted with 1 or 2 substituents, an oxamoyl group optionally substituted with 1 or 2 substituents, an amino group optionally substituted with 1 or 2 substituents, a 4- to 7-membered alicyclic heterocyclic group optionally substituted with 1 or 2 substituents, a phenyl group optionally substituted with 1 to 3 substituents, or a 5- or 6-membered aromatic heterocyclic group optionally substituted with 1 to 3 substituents; and R2 represents hydrogen atom, a halogeno group, or the like.
Improved syntheses of fluorinated tertiary butylamines
Ok, Dong,Fisher, Michael H.,Wyvratt, Matthew J.,Meinke, Peter T.
, p. 3831 - 3834 (2007/10/03)
Two new and efficient methods using cyclic sulfamidate and nitrone chemistry were developed for the synthesis of the sterically congested 1,1- dimethyl-2-fluoroethylamine (1), 2-fluoro-1-(fluoromethyl)-1-methylethylamine (2) and 2-fluoro-1,1-bis-(fluoromethyl)-ethylamine (3).
Bicyclic and tricyclic compounds with β-amino alcohol groups as chiral ligands in the enantioselective reaction of diethylzinc with aldehydes
Aurich, Hans Guenter,Biesemeier, Frank,Geiger, Michael,Harms, Klaus
, p. 423 - 434 (2007/10/03)
Reduction of the enantiomerically pure allyloxy esters 9 or ent-9 with DIBAH afforded the corresponding aldehydes which were treated in situ with chiral or achiral hydroxylamines 8 to give nitrones 10. These underwent a spontaneous intramolecular 1,3-dipolar cycloaddition, affording the bicyclic compounds 11 and 12, respectively. In an analogous manner, a mixture of the tricyclic compounds 14 and 15 was prepared. Treatment of compound 16 with cyclohexene oxide afforded a mixture of diastereomers 17 and 18. Diastereomers 14 and 15 as well as 17 and 18 could be separated by chromatography. X-ray analyses of compounds 11Ff, 17, and 11Af · HCI were performed. The bicyclic and tricyclic compounds were used as chiral ligands in the reaction of diethylzinc with aldehydes 19, in particular with benzaldehyde (19a). Using bicyclic compounds with a tertiary β-hydroxyalkyl substituent at the N atom as ligands, ee's in the range of 78 to 95% were found. Whereas for the best ligands 11Ae and Af the enantioselectivity in the reaction of 4-tolualdehyde was only slightly decreased, with the aliphatic aldehydes 19c and d distinctly lower enantioselectivities were determined. VCH Verlagsgesellschaft mbH, 1997.
22563-90-2 Process route
-
-
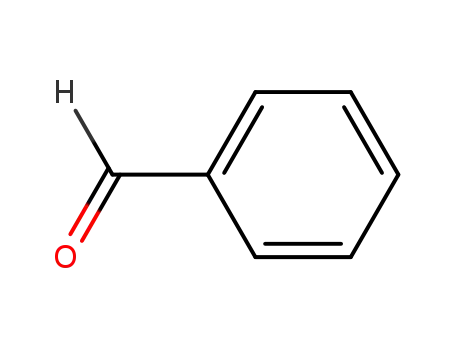
100-52-7
benzaldehyde

-
-
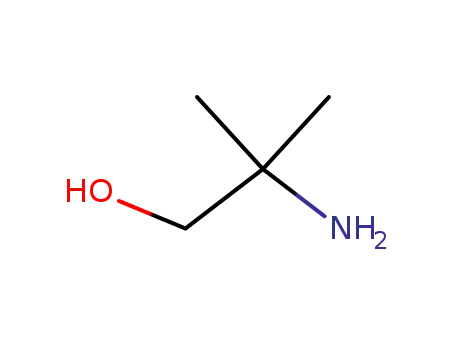
124-68-5
2-Amino-2-methyl-1-propanol

-
-
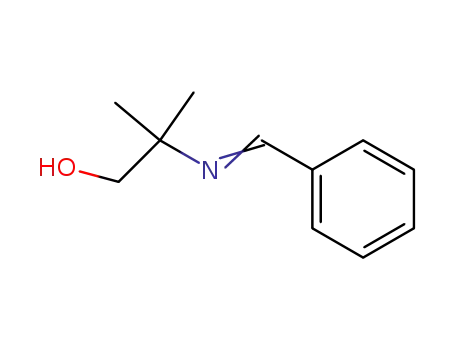
22563-90-2,148202-03-3
2-Benzalamino-isobutanol
| Conditions | Yield |
|---|---|
|
In
ethanol;
for 2h;
Ambient temperature;
|
85% |
|
With
4 A molecular sieve; iodine;
In
toluene;
Ambient temperature;
|
56% |
|
In
methanol;
|
|
|
With
magnesium sulfate;
In
diethyl ether;
for 18h;
Ambient temperature;
|
|
|
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
for 5h;
Heating;
|
|
|
toluene-4-sulfonic acid;
In
benzene;
for 4h;
Heating / reflux;
|
|
|
With
sodium sulfate;
In
methanol;
|
-
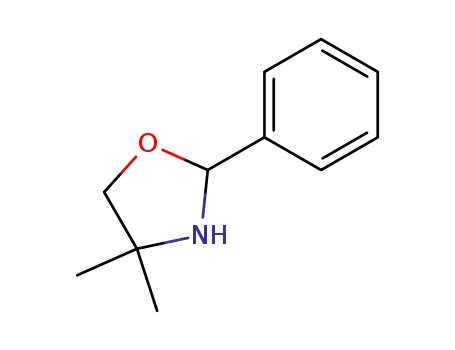
-
20515-61-1
2-phenyl-4,4-dimethyloxazolidine

-

-
22563-90-2,148202-03-3
2-Benzalamino-isobutanol
| Conditions | Yield |
|---|---|
|
In
tetrachloromethane;
at 35 ℃;
Equilibrium constant;
various solvents;
|
|
|
at 22 ℃;
Equilibrium constant;
various solvents;
|
22563-90-2 Upstream products
-
20515-61-1

2-phenyl-4,4-dimethyloxazolidine
-
100-52-7

benzaldehyde
-
124-68-5

2-Amino-2-methyl-1-propanol
22563-90-2 Downstream products
-
20515-61-1

2-phenyl-4,4-dimethyloxazolidine
-
10250-27-8
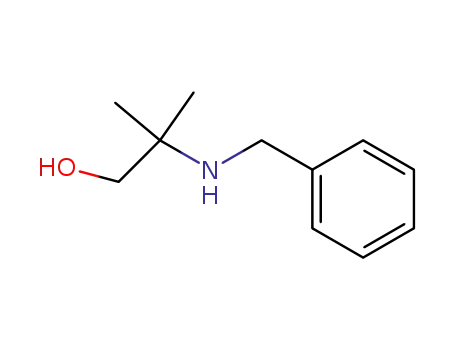
2-benzylamino-2-methyl-1-propanol
-
127535-98-2
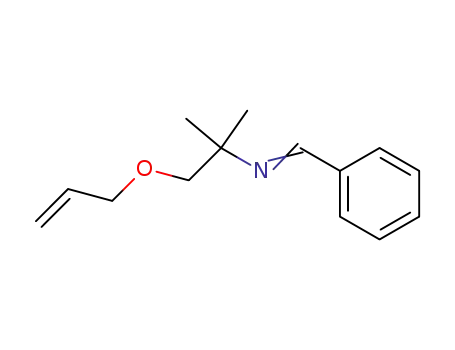
(2-Allyloxy-1,1-dimethyl-ethyl)-[1-phenyl-meth-(E)-ylidene]-amine
-
55277-95-7
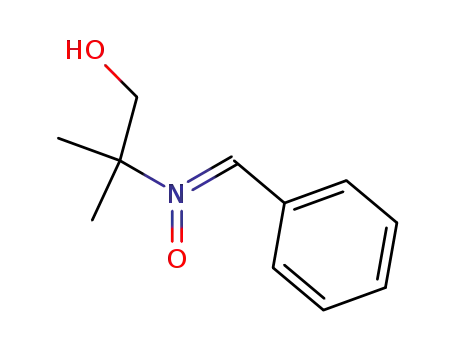
N-benzylidene-1-hydroxy-2-methyl-2-propanamine N-oxide








