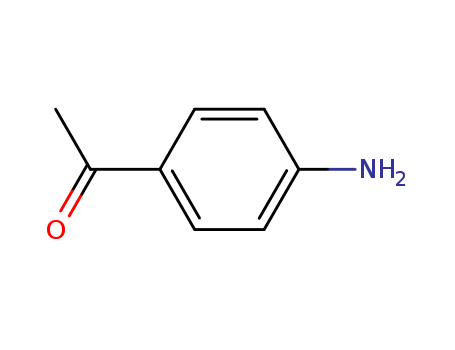
Product Details
99-92-3 Properties
- Molecular Formula:C8H9NO
- Molecular Weight:135.166
- Appearance/Colour:slightly yellow to brown crystalline powder
- Vapor Pressure:0.00158mmHg at 25°C
- Melting Point:103-107 °C(lit.)
- Refractive Index:1.571
- Boiling Point:294.8 °C at 760 mmHg
- PKA:2.17±0.10(Predicted)
- Flash Point:132.1 °C
- PSA:43.09000
- Density:1.096 g/cm3
- LogP:2.05260
99-92-3 Usage
Description
4-Aminoacetophenone is in the form of yellow needle-like crystals, with a melting point of 106°C, a boiling point of 293-295°C, and 195-200°C (2kPa). Soluble in hot water, soluble in alcohol, ether, dilute hydrochloric acid and dilute sulfuric acid, it is slightly soluble in benzene and cold water. Has a special pleasant fragrance. It can be used as a pharmaceutical intermediate.
Synthesis
Through a Williamson ether synthesis method and Smiles rearrangement reaction, benzamide compounds are produced, then the 4-aminoacetophenone preparation is finished after hydrolysis.
Applications
4′-Aminoacetophenone, is used in pharmaceuticals, medicines and other organic products. It is also used as a reagent for the photometric determination of Ce. As pharmaceutical intemediate or in the determination of palladium and vitaminB1.
Applications
A novel utilization of aminophenone derivative in toxicology, for treating intoxications with cyanogenic toxic substances.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. acids, Incompatible to Acid chlorides, Acid anhydrides, Chloroformates, Strong oxidizing agents, Strong reducing agents. Stable under ordinary conditions.
Chemical Properties
slightly yellow to brown crystalline powder
Uses
A novel utilization of aminophenone derivative in toxicology, for treating intoxications with cyanogenic toxic substances.
Uses
4'-Aminoacetophenone, is used in pharmaceuticals, medicines and other organic products. It is also used as a reagent for the photometric determination of Ce. As pharmaceutical intemediate or in the determination of palladium and vitaminB1.
Uses
4-Aminoacetophenone is a novel utilization of aminophenone derivative in toxicology used for treating intoxications with cyanogenic toxic substances.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 34, p. 2013, 1986 DOI: 10.1248/cpb.34.2013Tetrahedron Letters, 27, p. 4687, 1986 DOI: 10.1016/S0040-4039(00)85038-8
General Description
4′-Aminoacetophenone, commonly known as Clenbuterol Impurity D, is a related compound of sympathomimetic amine drug clenbuterol. Clenbuterol belongs to a group of compounds referred to as β-2-agonists and is generally used in the treatment of asthma.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Safety Profile
Poison by ingestion and intraperitoneal routes. When heated to decomposition it emits toxic fumes of NO,. See also AROMATIC AMINES
Purification Methods
It is soluble in hot H2O. UV (EtOH) has max 403nm (log 4.42) [Johnson J Am Chem Soc 75 2720 1953]. [Vandenbelt Anal Chem 26 726 1954.] The 2,4-dinitrophenylhydrazone has m 266-267o (from CHCl3 or EtOH) with 403nm (log 4.42), and the max semicarbazone has m 193-194o(dec)(from MeOH). The hydrochloride has m 98o(dec)(from H2O). [Beilstein 14 IV 100.]
Toxicity evaluation
Harmful if swallowed. Causes eye, skin, and respiratory tract irritation. May cause methemoglobinemia.
99-92-3 Relevant articles
Palladated halloysite hybridized with photo-polymerized hydrogel in the presence of cyclodextrin: An efficient catalytic system benefiting from nanoreactor concept
Sadjadi, Samahe,Atai, Mohammad
, (2019)
Considering the excellent performance of halloysite as a catalyst support and in an attempt to benefit from the concept of nanoreactors in the catalysis, an innovative catalytic system has been designed, in which acrylamide and bis-acrylamide were photo-p
Zinc-catalyzed reactions of ethenetricarboxylates with 2- (trimethylsilylethynyl)anilines leading to bridged quinoline derivatives
Yamazaki, Shoko,Morikawa, Satoshi,Miyazaki, Kazuya,Takebayashi, Masachika,Yamamoto, Yuko,Morimoto, Tsumoru,Kakiuchi, Kiyomi,Mikata, Yuji
, p. 2796 - 2799 (2009)
Zinc Lewis acid-catalyzed cyclization of thenetricarboxylate derivatives 1 with 2-ethynylanilines has been examined. Reaction of 1,1-diethyl 2-fert-butyl ethenetricarboxylate 1 b with 2-(trimethylsilylethynyl)aniline substrates In the presence of Zn(OTf)
Decatungstate-mediated solar photooxidative cleavage of CC bonds using air as an oxidant in water
Du, Dongdong,Luo, Junfei,Shi, Sanshan,Xie, Pan,Xue, Cheng
, p. 5936 - 5943 (2021/08/23)
With the increasing attention for green chemistry and sustainable development, there has been much interest in searching for greener methods and sources in organic synthesis. However, toxic additives or solvents are inevitably involved in most organic transformations. Herein, we first report the combination of direct utilization of solar energy, air as the oxidant and water as the solvent for the selective cleavage of CC double bonds in aryl olefins. Various α-methyl styrenes, diaryl alkenes as well as terminal styrenes are well tolerated in this green and sustainable strategy and furnished the desired carbonyl products in satisfactory yields. Like heterogeneous catalysis, this homogeneous catalytic system could also be reused and it retains good activity even after repeating three times. Mechanism investigations indicated that both O2- and 1O2 were involved in the reaction. Based on these results, two possible mechanisms, including the electron transfer pathway and the energy transfer pathway, were proposed.
Electrochemical reactivity of S-phenacyl-O-ethyl-xanthates in hydroalcoholic (MeOH/H2O 4:1) and anhydrous acetonitrile media
López-López, Ernesto Emmanuel,López-Jiménez, Sergio J.,Barroso-Flores, Joaquín,Rodríguez-Cárdenas, Esdrey,Tapia-Tapia, Melina,López-Téllez, Gustavo,Miranda, Luis D.,Frontana-Uribe, Bernardo A.
, (2021/04/12)
The electrochemical behavior of a series of S-phenacyl-O-ethyl-xanthates (O-ethyl-dithiocarbonate acetophenone derivatives) in hydroalcoholic (MeOH/H2O 4:1) and anhydrous media (ACN/TBAPF6) using carbon electrodes was studied. Cyclic voltammetry showed in hydroalcoholic media only two cathodic waves, whereas in ACN one anodic and two cathodic waves were present. The first cathodic wave corresponded to the reduction of the phenylketone group, whereas the first anodic was attributed to the xanthate unit. Macroelectrolysis on graphite and vitreous carbon at anodic and cathodic potentials, let us to explore the synthetic potential of this electrochemical reactions. With some compounds in hydroalcoholic media and using carbon electrodes, polymeric material was deposited on the electrode impeding the reaction; this deposit was characterized by AFM and SEM-EDS. The electroreduction on Ti electrode overcome this problem and gave the corresponding acetophenones (>95%). On the other hand, in ACN, small quantities of the dimeric 1,4-dicarbonyl compounds X-PhCOCH2CH2COPh-X (7–15%), as well as the corresponding acetophenones (ca. 50%) were isolated. Oxidation macroelectrolysis showed a very complicated transformation without synthetic value. The reaction mechanism for the reduction and the homolytic dissociation into the phenacyl radical was supported by DFT calculations.
Highly selective hydrogenation of aromatic ketones to alcohols in water: effect of PdO and ZrO2
Alsalahi, W.,Trzeciak, A. M.,Tylus, W.
, p. 10386 - 10393 (2021/08/09)
Pd/ZrO2and PdO/ZrO2composites, containing Pd or PdO nanoparticles, were prepared using an original one-step methodology. These nanocomposites catalyze the hydrogenation of acetophenone (AP) at 1 bar and 10 bar of H2in an aqueous solution. Compared to unsupported Pd or PdO nanoparticles, a remarkable increase in their activity was achieved as a result of interaction with zirconia. An unsupported PdO hydrogenated AP mainly to ethylbenzene (EB), while excellent regioselectivity towards 1-phenylethanol (PE) was obtained with PdO/ZrO2and it was preserved during recycling. Similarly, regioselectivity to PE was higher with Pd/ZrO2compared to unsupported Pd NPs. PdO and zirconia resulted in high selectivity to alcohols in the hydrogenation of substituted acetophenones.
Cu2O-CuO/Chitosan Composites as Heterogeneous Catalysts for Benzylic C?H Oxidation at Room Temperature
Kanarat, Jurin,Bunchuay, Thanthapatra,Klysubun, Wantana,Tantirungrotechai, Jonggol
, p. 4833 - 4840 (2021/10/07)
Recently, in catalysis, chitosan has been exploited as a macrochelating ligand for metal active species due to the presence of various functional groups in its structure. Moreover, copper-based catalysts are classified as one of the most environmentally friendly catalytic systems and their use for the oxidation of alkylarene has not been established much. Therefore, in this work, the hydrothermal synthesis of copper oxide-chitosan composites as heterogeneous catalysts for the benzylic C?H oxidation of alkylarene was investigated. Characterization results reveal mixed phases of CuO and Cu2O, inferring the ability of chitosan to act as a reducing sugar under the hydrothermal condition. The pre-existing interaction between copper species and chitosan as well as the co-existence of the Cu2O and CuO structures give rise to the efficient performance of the catalysts. The synthesized composites exhibit high activity for the oxidation of fluorene to 9-fluorenone at room temperature and small catalyst loading (1 mol % of Cu, >90 % conversion and 100 % selectivity). Superior TOF was observed, and a good scope of substrates can be converted to corresponding ketones in 48–97 % yields with these copper oxide-chitosan catalysts. In addition, the catalysts can be used for up to nine cycles without significant decrease of the activity.
99-92-3 Process route
-
![1-[4-nitrophenyl]-1-ethanol](/upload/2023/2/8a1d7fce-6377-47b9-9219-a3bbf0d15d53.png)
-
6531-13-1
1-[4-nitrophenyl]-1-ethanol

-
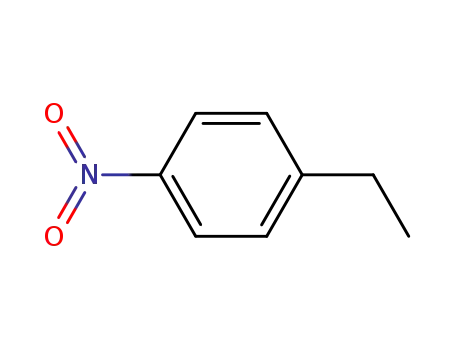
-
100-12-9,94234-68-1,34527-20-3
4-ethylnitrobenzene

-
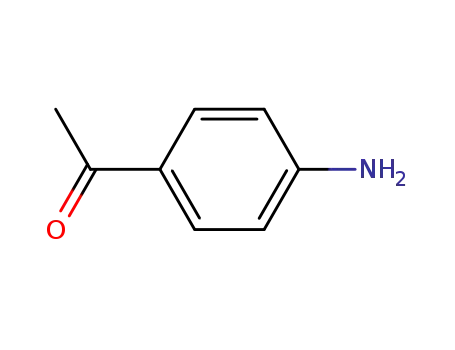
-
99-92-3
p-aminobenzophenone
| Conditions | Yield |
|---|---|
|
With
palladium dichloride;
In
methanol;
at 40 ℃;
for 24h;
chemoselective reaction;
Inert atmosphere;
Green chemistry;
|
-
![4-(2-methyl-[1,3]dioxolan-2-yl)-phenylamine](/upload/2023/2/06c83859-2349-43ec-96c9-e6b46046f6bd.png)
-
19073-16-6
4-(2-methyl-[1,3]dioxolan-2-yl)-phenylamine

-
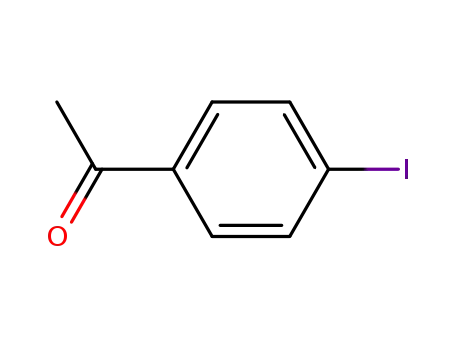
-
13329-40-3
4-Iodoacetophenone

-

-
99-92-3
p-aminobenzophenone
| Conditions | Yield |
|---|---|
|
With
N-iodo-succinimide; sodium nitrite;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 4h;
|
31% 62% |
99-92-3 Upstream products
-
917-64-6
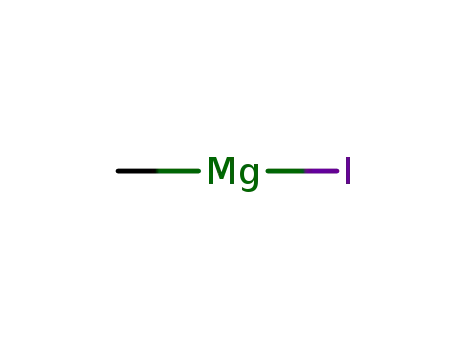
methyl magnesium iodide
-
2835-68-9
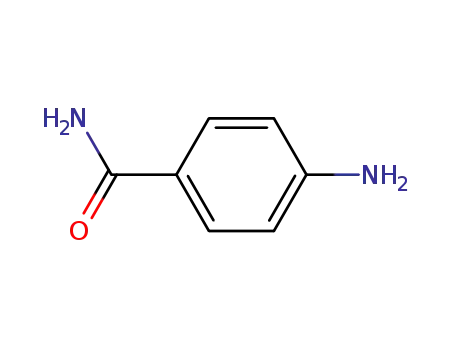
4-aminobenzamide
-
1563-87-7
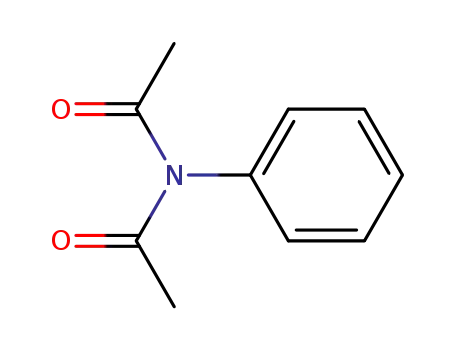
N-acetyl-N-phenylacetamide
-
108-24-7
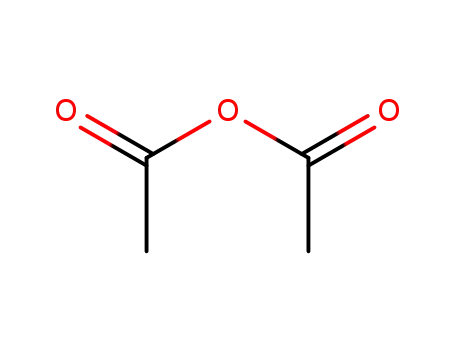
acetic anhydride
99-92-3 Downstream products
-
1197-55-3
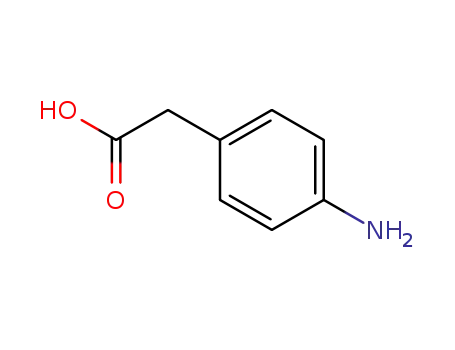
4-aminophenylacetic acid
-
5325-89-3
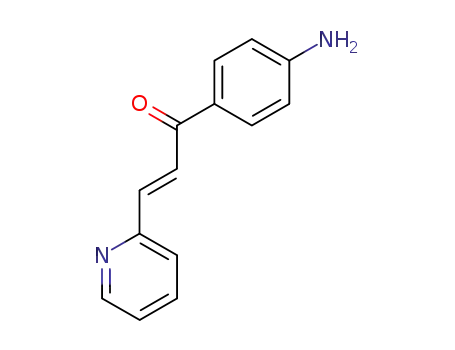
(E)-1-(4′-aminophenyl)-3-(pyridin-2-yl)prop-2-en-1-one
-
24870-12-0
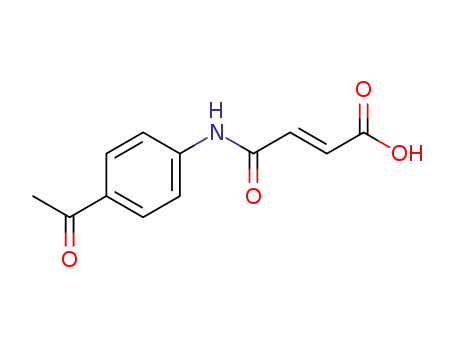
N-(4-acetyl-phenyl)-maleamic acid
-
40101-59-5
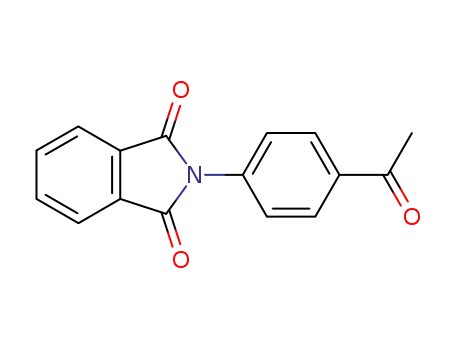
2-(4-acetylphenyl)-1H-isoindole-1,3(2H)-dione








