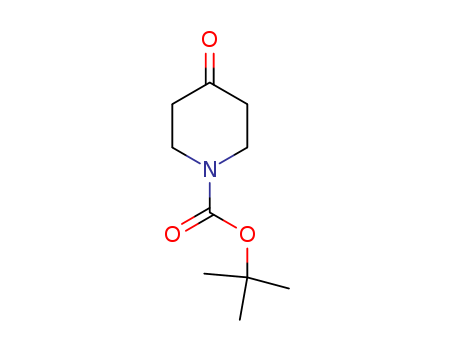
N-(tert-Butoxycarbonyl)-4-piperidone 79099-07-3
- CasNo:79099-07-3
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
79099-07-3 Properties
- Molecular Formula:C10H17NO3
- Molecular Weight:199.25
- Appearance/Colour:light yellow powder
- Vapor Pressure:0.00215mmHg at 25°C
- Melting Point:73-77 ºC
- Refractive Index:1.481
- Boiling Point:289.8 ºC at 760 mmHg
- PKA:-1.58±0.20(Predicted)
- Flash Point:129.1 ºC
- PSA:46.61000
- Density:1.099 g/cm3
- LogP:1.52430
79099-07-3 Usage
Uses
1-t-Boc-4-piperidone is a compound useful in organic synthesis.
Chemical Properties
Light Yellow Powder
Uses
1-Boc-4-piperidone is used as a pharmaceutical intermediate. It is involved in the preparation of 1-piperidin-4-yl-substituted butyro- and valerolactams.
Uses
1-t-Boc-4-piperidone is a compound useful in organic synthesis used in the preparation of (aminoaryl)(benzyloxy)pyridines as potential antitumor agents.
Application
N-(tert-Butoxycarbonyl)-4-piperidone is a chemical intermediate that belongs to the group of p2. It is an ancillary used in the synthesis of many organic compounds, including epidermal growth factor analogs. It has been shown to have pharmacokinetic properties that are similar to those of epidermal growth factor and can be used for the treatment of cancer. This drug has been shown to inhibit the growth of skin cancer cells and may also be useful in treating other types of cancer.
Uses
1-t-Boc-4-piperidone is a compound useful in organic synthesis.
Chemical Properties
Light Yellow Powder
Uses
1-Boc-4-piperidone is used as a pharmaceutical intermediate. It is involved in the preparation of 1-piperidin-4-yl-substituted butyro- and valerolactams.
Uses
1-t-Boc-4-piperidone is a compound useful in organic synthesis used in the preparation of (aminoaryl)(benzyloxy)pyridines as potential antitumor agents.
Application
N-(tert-Butoxycarbonyl)-4-piperidone is a chemical intermediate that belongs to the group of p2. It is an ancillary used in the synthesis of many organic compounds, including epidermal growth factor analogs. It has been shown to have pharmacokinetic properties that are similar to those of epidermal growth factor and can be used for the treatment of cancer. This drug has been shown to inhibit the growth of skin cancer cells and may also be useful in treating other types of cancer.
InChI:InChI=1/C10H17NO3/c1-10(2,3)14-9(13)11-6-4-8(12)5-7-11/h4-7H2,1-3H3
79099-07-3 Relevant articles
Synthesis of (R)-(+)-1,2,3,6-Tetrahydro-4-[U-14C]phenyl-1-[(3-phenyl-3-cyclohexenyl-1 -yl)methyl]pyridine, a potential antipsychotic agent
Ekhato,Huang
, p. 57 - 63 (1995)
1,2,3,6-Tetrahydro-4-[U-14C]phenylpyridine (12) was synthesized and coupled to (R)-3-phenyl-3-cyclohexene-1-carboxylic acid to make the titled compound 7 in 21% overall yield. Purification considerations were an important factor in the choice of a reaction sequence to 7, and successful synthesis was facilitated by the superiority of BF3.OEt2 as dehydrating reagent in this system. This otherwise problematic sequence offers an efficient and simple route to compound 7 (PD 143188).
A Convenient Synthesis and Biological Research of Novel 5,6,7,8-Tetrahydro-1,6-naphthyridin-2(1H)-one Derivatives Hydrochloride as Cytotoxic Agents
Xu, Guiqing,Gao, Yuan,Sun, Bin,Peng, Lizeng,Mao, Longfei,Jiang, Yuqin,Ding, Qingjie
, p. 2151 - 2156 (2018)
A series of 5,6,7,8-tetrahydro-1,6-naphthyridin-2(1H)-one derivatives hydrochloride were obtained using a convenient and mild method from 4-piperidone monohydrate hydrochloride. The newly synthesized compounds and their derivatives were characterized by 1H NMR, 13C NMR, and high-resolution mass spectrometry. Furthermore, cytotoxicity in vitro of the synthesized compounds were screened using MTT or CCK8 assay. The results showed that some of the compounds showed potential antitumor activity. Among of them, compound 10a had effects against tumor cells (MOLM-13), and the half maximal inhibitory concentration value was 76?μmol/L.
ANTI-CD25 ANTIBODY-MAYTANSINE CONJUGATES AND METHODS OF USE THEREOF
-
Paragraph 00402-00405, (2021/04/10)
The present disclosure provides anti-CD25 antibody-maytansine conjugate structures. The disclosure also encompasses methods of production of such conjugates, as well as methods of using the same.
ANTI-CD37 ANTIBODY-MAYTANSINE CONJUGATES AND METHODS OF USE THEREOF
-
Paragraph 00409-00412, (2021/05/07)
The present disclosure provides anti-CD37 antibody-maytansine conjugate structures. The disclosure also encompasses methods of production of such conjugates, as well as methods of using the same.
A general N-alkylation platform via copper metallaphotoredox and silyl radical activation of alkyl halides
Cabré, Albert,Dow, Nathan W.,MacMillan, David W. C.
supporting information, p. 1827 - 1842 (2021/07/07)
The catalytic union of amides, sulfonamides, anilines, imines, or N-heterocycles with a broad spectrum of electronically and sterically diverse alkyl bromides has been achieved via a visible-light-induced metallaphotoredox platform. The use of a halogen abstraction-radical capture (HARC) mechanism allows for room temperature coupling of C(sp3)-bromides using simple Cu(II) salts, effectively bypassing the prohibitively high barriers typically associated with thermally induced SN2 or SN1 N-alkylation. This regio- and chemoselective protocol is compatible with >10 classes of medicinally relevant N-nucleophiles, including established pharmaceutical agents, in addition to structurally diverse primary, secondary, and tertiary alkyl bromides. Furthermore, the capacity of HARC methodologies to engage conventionally inert coupling partners is highlighted via the union of N-nucleophiles with cyclopropyl bromides and unactivated alkyl chlorides, substrates that are incompatible with nucleophilic substitution pathways. Preliminary mechanistic experiments validate the dual catalytic, open-shell nature of this platform, which enables reactivity previously unattainable in traditional halide-based N-alkylation systems.
Cobalt-Catalyzed Aerobic Oxidative Cleavage of Alkyl Aldehydes: Synthesis of Ketones, Esters, Amides, and α-Ketoamides
Li, Tingting,Hammond, Gerald B.,Xu, Bo
supporting information, p. 9737 - 9741 (2021/05/31)
A widely applicable approach was developed to synthesize ketones, esters, amides via the oxidative C?C bond cleavage of readily available alkyl aldehydes. Green and abundant molecular oxygen (O2) was used as the oxidant, and base metals (cobalt and copper) were used as the catalysts. This strategy can be extended to the one-pot synthesis of ketones from primary alcohols and α-ketoamides from aldehydes.
79099-07-3 Process route
-
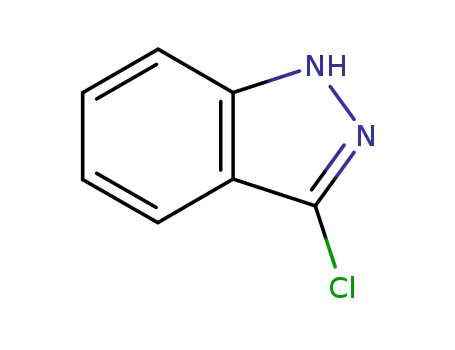
-
29110-74-5
3-chloroindazole

-
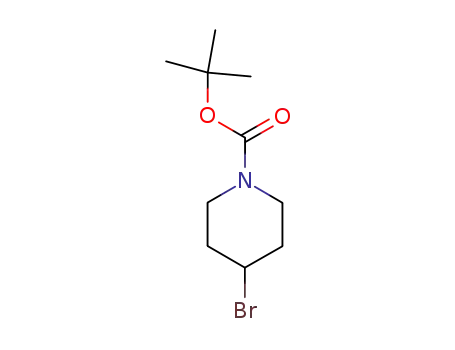
-
180695-79-8
tert-butyl 4-bromo-1-piperidinecarboxylate

-
-
C17H22ClN3O2

-
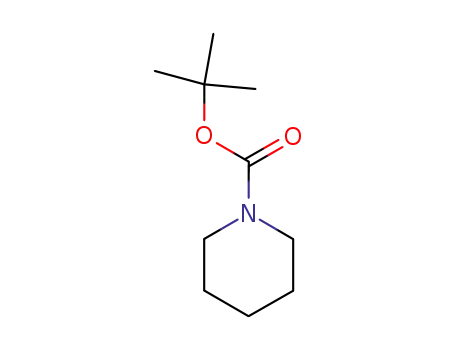
-
75844-69-8
t-butyl piperidinecarboxylate

-
-
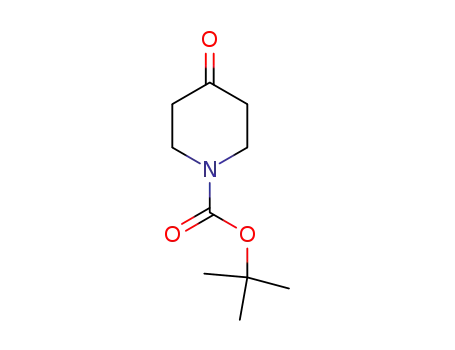
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
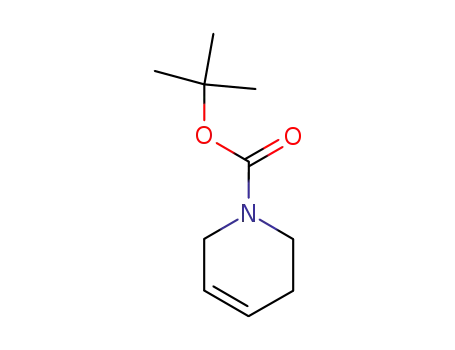
-
85838-94-4
N-Boc-1,2,3,6-tetrahydropyridine
| Conditions | Yield |
|---|---|
|
With
copper acetylacetonate; 1,1,1,3,3,3-hexamethyl-2-(trimethylsilyl)-2-trisilanol; [Ir(3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl)2(4,4'-bis(trifluoromethyl)bipyridine)]PF6; N,N,N',N'-tetramethylguanidine;
In
acetonitrile;
at 20 ℃;
for 18h;
regioselective reaction;
Irradiation;
Sealed tube;
|
23% |
-
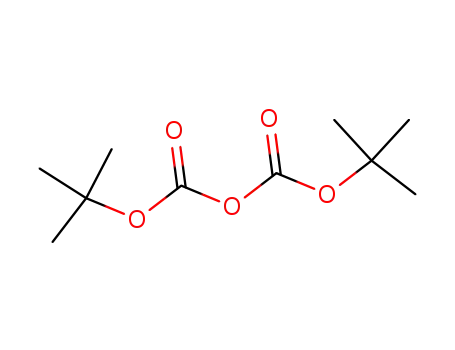
-
24424-99-5
di-tert-butyl dicarbonate

-
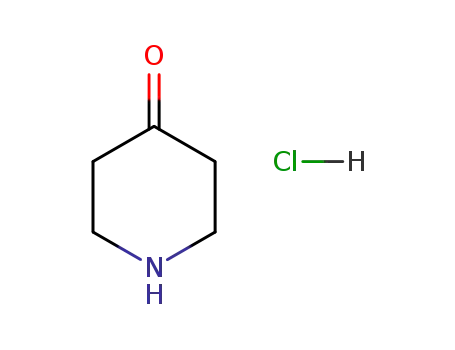
-
41979-39-9
4-piperidone hydrochloride

-

-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
water;
1) 35 deg C, 1 h; 2) 50 deg C, 2.5 h;
|
100% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 20 ℃;
for 16h;
|
100% |
|
di-tert-butyl dicarbonate; 4-piperidone hydrochloride;
With
triethylamine;
dmap;
In
methanol;
at 20 ℃;
for 20h;
With
hydrogenchloride;
In
dichloromethane;
|
100% |
|
In
1,4-dioxane; water;
at 20 ℃;
for 4h;
|
100% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran;
at 25 ℃;
for 6h;
|
98% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 24h;
|
98% |
|
With
triethylamine;
In
1,4-dioxane; water;
at 0 - 20 ℃;
Concentration;
|
95% |
|
With
sodium hydrogencarbonate;
In
water; acetone;
at 20 ℃;
for 24h;
|
94% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
at 20 - 70 ℃;
|
93% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 20 ℃;
for 2h;
Inert atmosphere;
|
93% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 20 ℃;
for 16h;
|
93% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 ℃;
for 0.5h;
|
92.5% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 0.8h;
|
92% |
|
4-piperidone hydrochloride;
With
triethylamine;
In
tetrahydrofuran;
for 0.0833333h;
di-tert-butyl dicarbonate;
With
dmap;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
91.8% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
for 24h;
Ambient temperature;
|
90% |
|
4-piperidone hydrochloride;
With
sodium hydroxide;
In
water;
at 20 - 30 ℃;
for 0.333333h;
di-tert-butyl dicarbonate;
In
water;
at 20 - 30 ℃;
for 12h;
Concentration;
Reagent/catalyst;
|
90% |
|
With
1,4-dioxane; triethylamine;
In
water;
at 20 ℃;
|
89% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 16h;
|
88% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 0 - 25 ℃;
|
87.44% |
|
With
sodium carbonate;
In
1,4-dioxane; water;
at 20 ℃;
for 1h;
|
87% |
|
With
sodium carbonate;
In
1,4-dioxane; water;
at 20 ℃;
for 1h;
|
87% |
|
With
sodium carbonate;
In
1,4-dioxane; water;
at 20 ℃;
for 1h;
|
87% |
|
With
sodium carbonate;
In
1,4-dioxane; water;
at 20 ℃;
for 1h;
|
87% |
|
With
sodium carbonate;
In
1,4-dioxane;
at 20 ℃;
|
85% |
|
With
sodium carbonate;
In
water;
at 35 - 50 ℃;
for 4h;
|
81% |
|
With
N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water;
at 20 ℃;
for 24h;
|
80.2% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 16h;
|
79% |
|
4-piperidone hydrochloride;
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 0.5h;
di-tert-butyl dicarbonate;
In
dichloromethane;
at 20 ℃;
for 16h;
|
79% |
|
4-piperidone hydrochloride;
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
di-tert-butyl dicarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 12h;
|
78% |
|
With
triethylamine;
In
methanol; dichloromethane;
at 0 - 20 ℃;
for 16h;
|
78% |
|
With
N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water;
at 20 ℃;
for 24h;
|
75% |
|
With
N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane;
at 20 ℃;
for 24h;
|
75% |
|
With
triethylamine;
In
methanol;
at 20 ℃;
for 2h;
|
75% |
|
With
triethylamine;
In
tetrahydrofuran; water;
for 2h;
Ambient temperature;
|
74.4% |
|
With
N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water;
at 20 ℃;
|
73.7% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 10 - 20 ℃;
|
65% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
for 18h;
Ambient temperature;
|
63% |
|
With
sodium hydrogencarbonate;
In
water; acetonitrile;
at 20 ℃;
|
62% |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 6h;
|
40% |
|
With
sodium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
10.9 g |
|
With
sodium hydrogencarbonate; sodium chloride;
In
tetrahydrofuran; water;
at 20 ℃;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 20 ℃;
|
|
|
With
potassium carbonate; triethylamine;
In
chloroform;
at 0 - 20 ℃;
|
|
|
With
sodium hydrogencarbonate; sodium chloride;
In
tetrahydrofuran; water;
at 20 ℃;
|
|
|
With
triethylamine;
In
methanol;
at 20 ℃;
for 2h;
|
|
|
4-piperidone hydrochloride;
With
triethylamine;
In
dichloromethane;
for 0.0833333h;
di-tert-butyl dicarbonate;
With
dmap;
In
dichloromethane;
at 25 ℃;
for 20h;
|
3.9 g |
|
With
sodium hydroxide;
|
|
|
With
sodium hydroxide; sodium monohydrogen sulfate;
In
tetrahydrofuran;
|
|
|
With
triethylamine;
In
dichloromethane;
at 10 - 30 ℃;
for 16.25h;
|
79099-07-3 Upstream products
-
41661-47-6
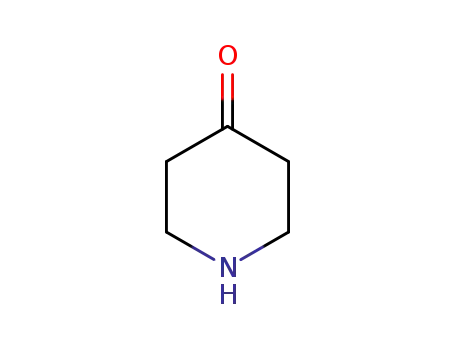
piperidin-4-one
-
24424-99-5

di-tert-butyl dicarbonate
-
41979-39-9

4-piperidone hydrochloride
-
40064-34-4
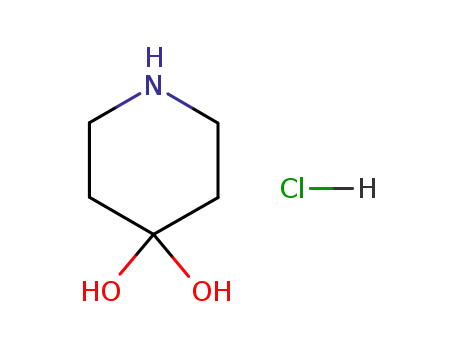
piperidine-4,4-diol hydrochloride
79099-07-3 Downstream products
-
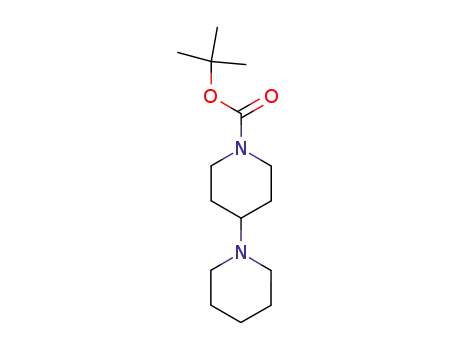
125541-12-0
tert-butyl 1,4’-bipiperidine-1’-carboxylate
-
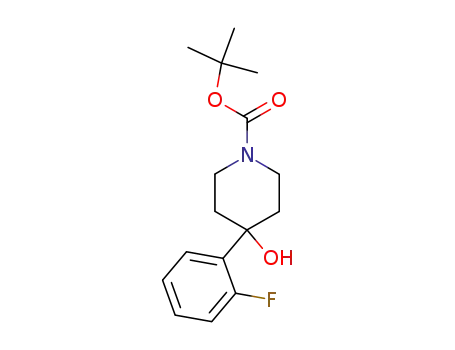
403806-35-9
4-(2-fluoro-phenyl)-4-hydroxypiperidine-1-carboxylic acid tert-butyl ester
-
235109-63-4
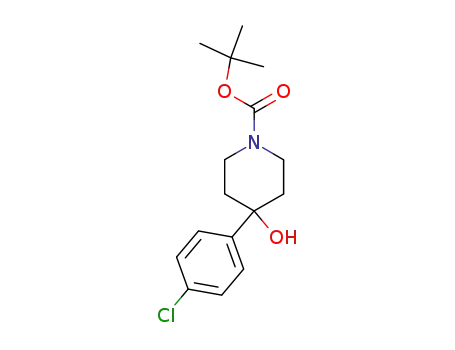
4-(4-chloro-phenyl)-4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester
-
302924-67-0
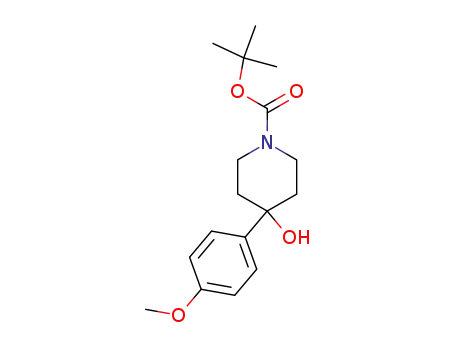
tert-butyl 4-hydroxy-4-(4-methoxyphenyl)-1-piperidinecarboxylate








