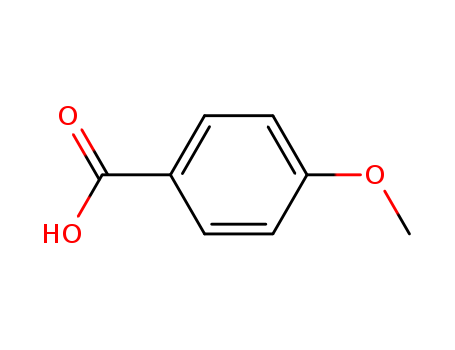
Product Details
100-09-4 Properties
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:White powder
- Vapor Pressure:0.002mmHg at 25°C
- Melting Point:181-186 °C
- Refractive Index:1.571-1.576
- Boiling Point:278.305 °C at 760 mmHg
- PKA:4.50(at 25℃)
- Flash Point:115.46 °C
- PSA:46.53000
- Density:1.208 g/cm3
- LogP:1.39340
100-09-4 Usage
Description
4-methoxybenzoic acid, also known as p-Anisic acid or draconic acid, is an organic acid with a sweet flavor. It is one of the isomers(m-anisic acid, and o-anisic acid) of anisic acid. The term "anisic acid" often refers to this form specifically. P-anisic acid is produced through the oxidation of p-cresyl-methyl ether.
Chemical Properties
A colorless needle crystal at room temperature, soluble in ethanol, ether, chloroform, slightly soluble in hot water, insoluble in cold water. It is used as intermediate of aniracetam, and also used as fragrance and preservative.
Uses
4-Methoxybenzoic acid (p-Anisic acid) is used in oxidation and reduction of cytochrome c in solution through different self-assembled monolayers on gold electrodes using cyclic voltammetry. p-Anisic acid has antiseptic properties. It is also used as an intermediate in the preparation of more complex organic compounds.
Preparation
4-Methoxybenzoic acid, also known as?p-Anisic acid,?is obtained by changing the mole ratio of cobalt and manganese and then using p-methoxy toluene with oxygen or oxygen containing gas in the presence of acetic acid.The experimental procedure is as follows:Using n-hexyl bromide, tri (n-hexyl) amine, para-methoxy toluene with cobalt chloride hexahydrate in about 9h;By a catalytic oxidation process using p-methoxy toluene and propionic acid over a catalyst comprising of CoBr2.6H2O and MnBr2.4H2O with a reaction time of 20h;Through changing the mole ratio of cobalt and manganese, using p-methoxy toluene with oxygen or oxygen containing gas in the presence of acetic acid to obtain the product.
Definition
ChEBI: 4-methoxybenzoic acid is a methoxybenzoic acid substituted with a methoxy group at position C-4. It has a role as a plant metabolite. It is functionally related to a benzoic acid. It is a conjugate acid of a 4-methoxybenzoate.
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 25, p. 973, 1988 DOI: 10.1002/jhet.5570250351Tetrahedron Letters, 34, p. 4603, 1993 DOI: 10.1016/S0040-4039(00)60635-4
General Description
4-Methoxybenzoic acid is the sole source of carbon and energy for growth in the cultures of Nocardia sp. DSM 1069. It is effective in clearing congestion in the lungs and the respiratory tracts in conditions like asthma or bronchitis.
Safety Profile
Poison by subcutaneous route.When heated to decomposition it emits acrid smoke andirritating vapors.
Purification Methods
Crystallise p-anisic acid from EtOH, water, EtOH/water or toluene. The S-benzylisothiuronium salt has m 189o (from EtOH). [Beilstein 10 II 91, 10 III 280, 10 IV 346.]
References
[1] Michael Ash (2004) Handbook of Preservatives[2] Asim Kumar Mukhopadhyay (2004) Industrial Chemical Cresols and Downstream Derivatives[3] https://en.wikipedia.org/wiki/P-Anisic_acid
InChI:InChI=1/C8H8O3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3,(H,9,10)
100-09-4 Relevant articles
Application of the Savage-Wood Treatment to the Quantitative Analysis of Kinetic Solvent Effects in Highly Aqueous Binary Solutions
Blokzijl, Wilfried,Jager, Jan,Engberts, Jan B. F. N.,Blandamer, Michael J.
, p. 6411 - 6413 (1986)
-
Lithium hypochlorite-clorox as a novel oxidative mixture for methyl ketones and methyl carbinols
Madler,Klucik,Soell,Brown,Liu,Berlin,Benbrook,Birckbichler,Nelson
, p. 230 - 234 (1998)
-
A new stilbene glucoside from a Chinese crude drug 'Heshouwu', (root of Polygonum multiflorum Thunb) (Japanese)
Hata,Kozawa,Baba
, p. 211 - 213 (1975)
-
-
Callow,Hill
, p. 844,847 (1937)
-
Facile electrochemical transformation of diazonium salts into carboxylic acids
Otero, M. Dolores,Batanero, Belen,Barba, Fructuoso
, p. 8215 - 8216 (2006)
The electrolyses of aryldiazonium tetrafluoroborates in dry DMF and Bu4NHSO4 as solvent-supporting electrolyte system, in the presence of CO2 led to the corresponding arylcarboxylic acids in very good yields.
-
Taylor
, p. 2018,2026 (1931)
-
A simple, one-pot oxidative esterification of aryl aldehydes through dialkyl acetal using hydrogen peroxide
Devarajan,Vijayakumar,Ramalingam,Vijayaraghavan
, p. 5849 - 5858 (2016)
A simple and an efficient one-pot procedure has been developed to synthesize various aryl carboxylic esters directly from aryl aldehydes using hydrogen peroxide without any catalyst. The reaction proceeds smoothly at room temperature. A preliminary investigation suggests the formation of dialkyl acetal as an intermediate during the reaction sequence.
Diphenyl disulfide and sodium in NMP as an efficient protocol for in situ generation of thiophenolate anion: Selective deprotection of aryl alkyl ethers and alkyl/aryl esters under nonhydrolytic conditions
Chakraborti, Asit K.,Nayak, Mrinal K.,Sharma, Lalima
, p. 1776 - 1780 (2002)
Aryl methyl ethers, methyl esters, aryl esters, and aryl sulfonates are chemoselectively deprotected under nonhydrolytic conditions by treatment with Ph2S2 (0.6 equiv) and Na (1.6 equiv) in NMP under reflux or at 90°C. Quantitative utilization of the 'PhS' moiety as the effective nucleophilic species represents conservation of atom economy. Other solvents such as HMPA, DMPU, DMEU, and DMF afforded comparable results. Chloro, nitro, aldehyde, α,α-diketone, and α,β-unsaturated ketone functionalities remain unaffected. The deprotection was found to take place in the order aryl ester > alkyl ester > aryl alkyl ether. Substrates bearing strong electron-withdrawing groups react at a faster rate than those not having such substitution. The differences in rate of reaction has been exploited for selective deprotection for intramolecular competition. An aryl acetate/benzoate is deprotected selectively in preference to a methyl ester or aryl methyl ether. Selective deprotection of a methyl ester is observed in the presence of an aryl alkyl ether.
Bromate exchange resin as an oxidizing agent in organic synthesis
Chetri, Ajay B.,Kalita, Biswajit,Das, Pranab J.
, p. 3317 - 3319 (2000)
Bromate exchange resin has been prepared by a simple elution technique and used for the oxidation of aromatic aldehydes to the carboxylic acids. Oxidation is carded out under biphasic condition. Work up is simple. Resin immobilized bromate ions have been used for the first time as an oxidizing agent in organic synthesis.
Homogeneous catalytic oxidation of styrene and styrene derivatives with hydrogen peroxide in the presence of transition metal-substituted polyoxotungstates
Duarte, Tiago A. G.,Estrada, Ana C.,Simes, Mrio M. Q.,Santos, Isabel C. M. S.,Cavaleiro, Ana M. V.,Neves, M. Graca P. M. S.,Cavaleiro, Jos A. S.
, p. 351 - 363 (2015)
The tetrabutylammonium (TBA) salts of the Keggin-type polyoxotungstates with general formula [XW11M(H2O)O39](n-m)-, where X = P, B or Si and M = Mn, Fe or Co, were evaluated as catalysts in the oxidation of styrene, α-methylstyrene, p-methylstyrene, α,p-dimethylstyrene, p-chlorostyrene, p-nitrostyrene, and p-methoxystyrene under mild conditions, using aqueous H2O2 as an eco-sustainable oxidant. In this study, the influence of the catalysts and of the different styrene substituents on the oxidation reaction profile was evaluated in terms of conversion and selectivity. For all the performed catalytic studies, the main product results from the oxidative cleavage of the vinyl double bond, except in the case of the oxidation of p-methoxystyrene catalysed by BW11Mn, for which p-methoxyphenol is the main product. The catalysts BW11Mn and SiW11Co give rise to 100% conversion for almost all of the substrates, excluding p-methoxystyrene and p-nitrostyrene for both catalysts and α,p-dimethylstyrene only in the case of BW11Mn. The selectivity for C=C cleavage products resulting from the oxidative cleavage of the vinyl double bond can be as high as 98%, reaching 98% conversion for p-nitrostyrene when SiW11Co was used as a catalyst. Possible pathways are discussed and the oxidation of a few presumed intermediates was carried out. The systematic study of several substituted styrene derivatives suggests a possible reactivity order for these compounds in the catalytic system considered.
-
Frimer et al.
, p. 4631,4632, 4634 (1977)
-
Mechanism of Solvolysis of Substituted Benzoyl Halides
Song, Byeong Doo,Jencks, William P.
, p. 8470 - 8479 (1989)
Most substituted benzoyl fluorides undergo hydrolysis in aqueous solution through an associative mechanism with ρ = 1.7, kHOH/kDOD = 2.3+/-0.2, little dependence on the leaving group (kCl/kF = 1.2), and general-base catalysis by fluoride ion.There is an abrupt change to a dissociative mechanism through an acylium ion intermediate for the hydrolysis of p-(dimethylamino)benzoyl and (in part) p-anisoyl fluorides, with ρ+ =/Cl/kF = 10E6-10E7, and kHOH/kDOD = 1.1 for p-Me2NPhCOF.Common ion inhibition by fluoride ion traps the p-(dimethylamino)benzoyl acylium ion, which undergoes hydration with kh ca. 10E9-10E10 s-1.The increase in the solvent isotope effect for hydrolysis of p-(dimethylamino)benzoyl fluoride to kHOH/kDOD = 1.9 in the presence of concentrated potassium fluoride is attributed to general-base-catalyzed hydration of the acylium ion intermediate.The large yield of trifluoroethyl ester from the solvolysis of p-anisoyl fluoride in TFE/EtOH/HOH suggests that the acylium ion reacts in a solvent-separated ion pair, with kh ca. 10E12 s-1; extrapolation predicts rate constants of =/> 10E13 s-1 for the hydration of less stable acylium ions.A change in sensitivity to solvent ionizing power from m = 1.4 in water to m = 0 in 60percent ethanol for p-(dimethylamino)benzoyl fluoride suggests a change to an associative mechanism.Benzoyl fluorides and acylium ions show selectivity toward alcohols, with βnuc ca. 0.2.The absence of common ion inhibition for the solvolysis of several benzoyl chlorides in water or 90percent TFE/HOH is consistent with kh >10E11 s-1 for the acylium ions.Solvolysis occurs through the dissociative reaction channel, with ρ+ = -3.0, even when the estimated lifetimes of the acylium ion species suggest that there is no chemical barrier for their hydration.However, there is a change to an associative mechanism for the solvolysis of p-nitrobenzoyl chloride in water.
Green and simple synthesis of p-anisic acid and other analogs from methyl paraben
Periyasamy, Senthil,Subbiah, Selvaraj
, p. 85 - 88 (2018)
Synthesis of p-anisic acid from commercially available methyl paraben was obtained in good yield and performed the each steps in shorter duration is reported. The E-factor was evaluated for each step was 3.0 and 2.30 respectively without transition metals content in the waste disposal. The solvents used in each steps were completely recovered and recycled in the consecutive batches. This methodology was applied to the synthesis of p-ethoxy benzoic acid and p-propyloxy benzoic acid and the other derivatives from methyl paraben obtained in good yield.
Alumina-mediated microwave thermolysis: A new approach to deprotection of benzyl esters
Varma, Rajender S.,Chatterjee, Arnab K.,Varma, Manju
, p. 4603 - 4606 (1993)
A simple and high yielding method for deprotection of benzyl esters is described which occurs under mild and solvent-free conditions on alumina surface using microwave irradiation.
Dithioester-enabled chemodivergent synthesis of acids, amides and isothiazoles via C[sbnd]C bond cleavage and C[sbnd]O/C[sbnd]N/C[sbnd]S bond formations under metal- and catalyst-free conditions
Soni, Sonam,Koley, Suvajit,Singh, Maya Shankar
, p. 2512 - 2516 (2017)
An operationally simple and user-friendly process to access privileged scaffolds such as acids, amides and isothiazoles has been devised employing β-ketodithioesters for the first time. Remarkably, the new protocol involves combination of C[sbnd]C bond cl
A Novel Fragmentation Reaction of α-(N-Siloxy)anilino Ketones induced by Fluoride Ions
Ohno, Masatomi,Ido, Motohisa,Eguchi, Shoji
, p. 1530 - 1531 (1988)
The reaction of α-(N-siloxy)anilino-substituted aromatic ketones with tetrabutylammonium fluoride afforded aromatic acids and aniline as the fragmentation products, presumably via ring rupture of the intermediate 1,2-oxazetidine.
Aquachlororuthenium(III) catalysis in the oxidation of substituted 4-oxo-4-arylbutanoic acids by bromate in acid medium: A kinetic and mechanistic study and validity of linear free-energy relationships
Manjari, Padma Sunitha,Reddy, Cherkupally Sanjeeva
, p. 707 - 719 (2011)
Ru(III) acts an efficient catalyst in the oxidation of substituted 4-oxo-4-arylbutanoic acids (4-oxo acids) by bromate in sulfuric acid medium, giving the corresponding benzoic acids in quantitative yields. The reaction shows first-order dependence in both [bromate] and [H2SO 4], and a non-linear dependence on both [oxo acid] and [catalyst]. Changing solvent from H2O to D2O increases the rate. The rate is not affected by ionic strength but decreases with increase in dielectric constant of the medium. Electron-releasing substituents in the phenyl ring of the substrate greatly accelerate the rate, whereas the retardation by electron-withdrawing substituents, though perceptible, is small. The linear free-energy relationship is characterized by smooth curves in Hammett plots of log k versus σ; however, linear plots are obtained with excellent correlation coefficients at all the studied temperatures, when Brown's σ+ values are used. The reaction constant is negative and decreases with increase in temperature. From the intersection of the lines in the Hammett and Arrhenius plots, the isokinetic relationship is evaluated. A mechanism involving a cyclic oxidant-substrate-catalyst ternary complex is proposed, in which both C-C bond-breaking and C-O bond formation are involved, and the oxidation state of Ru(III) remains unchanged. A rate law explaining all the kinetic results has been derived and verified. The reaction is an example of neighboring group participation in intramolecular catalysis and is potentially useful for the synthesis of substituted benzoic acids.
Catalytic C-H aerobic and oxidant-induced oxidation of alkylbenzenes (including toluene derivatives) over VO2+immobilized on core-shell Fe3O4?SiO2at room temperature in water
Mohammadpour, Pegah,Safaei, Elham
, p. 23543 - 23553 (2020)
Direct C-H bond oxidation of organic materials, and producing the necessary oxygenated compounds under mild conditions, has attracted increasing interest. The selective oxidation of various alkylbenzenes was carried out by means of a new catalyst containing VO2+ species supported on silica-coated Fe3O4 nanoparticles using t-butyl hydroperoxide as an oxidant at room temperature in H2O or solvent-free media. The chemical and structural characterization of the catalyst using several methods such as FTIR spectroscopy, XRD, FETEM, FESEM, SAED, EDX and XPS showed that VO2+ is covalently bonded to the silica surface. High selectivity and excellent conversion of various toluene derivatives, with less reactive aliphatic (sp3) C-H bonds, to related benzoic acids were quite noticeable. The aerobic oxygenation reaction of these alkylbenzenes was studied under the same conditions. All the results accompanied by sustainability of the inexpensive and simple magnetically separable heterogeneous catalyst proved the important criteria for commercial applications. This journal is
Palladium catalyzed carbonylation of iodoarenes in aqueous solubilized systems
Cheprakov, Andrei V.,Ponomareva, Natalia V.,Beletskaya, Irina P.
, p. 297 - 300 (1995)
Iodobenzene and substituted iodobenzenes can be easily carbonylated into benzoic acids under mild conditions, with simple palladium salts as catalysts and normal pressure of CO, by using aqueous microemulsions of the oil-in water kind as the reaction media.Surfactants of all three kinds, anionic, nonionic, and cationic, and simple aliphatic alcohols can be used to form the microemulsion media for carbonylation.The use of nonionic surfactants, the derivatives of polyethyleneglycol, is the most advantageous method as the surfactant is highly efficient in small amountswithout a cosurfactant because of its strong solubilizing ability.Keywords: Palladium; Carbon monoxide; Carbonylation; Water
4-Benzoyl-4-methylcyclohexa-2,5-dienone and its Benzoyl Substituted Derivatives: Isolated 4-Acylcyclohexa-2,5-dienones
Jackson, Lorraine B.,Waring, Anthony J.
, p. 857 - 858 (1985)
4-Benzoyl-4-methylcyclohexa-2,5-dienone and its 4-(4-chlorobenzoyl) and 4-(4-methoxybenzoyl) analogues (2a-c) have been isolated as the first simple examples of an elusive class of compounds, and their reactivities towards nucleophiles have been investigated.
Combining Oxoammonium Cation Mediated Oxidation and Photoredox Catalysis for the Conversion of Aldehydes into Nitriles
Nandi, Jyoti,Witko, Mason L.,Leadbeater, Nicholas E.
, p. 2185 - 2190 (2018)
A method to oxidize aromatic aldehydes to nitriles has been developed. It involves a dual catalytic system of 4-acetamido-TEMPO and visible-light photoredox catalysis. The reaction is performed using ammonium persulfate as both the terminal oxidant and nitrogen source.
Benzylic carbon oxidation by an in situ formed o-iodoxybenzoic acid (IBX) derivative
Ojha, Lawanya R.,Kudugunti, Shashi,Maddukuri, Padma P.,Kommareddy, Amitha,Gunna, Meena R.,Dokuparthi, Praveen,Gottam, Hima B.,Botha, Kiran K.,Parapati, Divya R.,Vinod, Thottumkara K.
, p. 117 - 121 (2009)
Benzylic C-H bonds are selectively oxidized to the corresponding carbonyl functionalities using catalytic quantities of 2-iodobenzoic acid (2IBAcid) and Oxone. The reported procedure tolerates different functional groups and operates under mild conditions. A radical mechanism is proposed for the transformation and evidence supporting the proposed mechanism is also presented. Georg Thieme Verlag Stuttgart.
Hydrothermal synthesis of platinum-group-metal nanoparticles by using HEPES as a reductant and stabilizer
So, Man-Ho,Ho, Chi-Ming,Chen, Rong,Che, Chi-Ming
, p. 1322 - 1331 (2010)
Platinum-group-metal (Ru, Os, Rh, Ir, Pd and Pt) nanoparticles are synthesized in an aqueous buffer solution of 4-(2-hydroxyethyl)-1-piper- azineethanesulfonic acid (HEPES) (200 mM, pH 7.4) under hydrothermal conditions (180 °C). Monodispersed (monodisper
A versatile route to the synthesis of 1-substituted β-carbolines by a single step Pictet-Spengler cyclization
Yang, Mei-Lin,Kuo, Ping-Chung,Damu, Amooru G.,Chang, Ren-Jie,Chiou, Wen-Fei,Wu, Tian-Shung
, p. 10900 - 10906 (2006)
A one-step conversion of l-tryptophan and activated aldehydes (1,2-dicarbonyl compounds) directly to 1-substituted β-carbolines without formation of the tetrahydro derivatives under modified Pictet-Spengler conditions was described. Moreover, a practical
-
Cahours
, (1845)
-
-
Rappoport,Gal
, p. 5246,5252 (1969)
-
Rapid chemoselective deprotection of benzyl esters by nickel boride
Khurana, Jitender M.,Arora, Reema
, p. 1127 - 1130 (2009)
Benzyl esters of a variety of acids can be chemoselectively cleaved on treatment with nickel boride in methanol at ambient temperature to give the parent carboxylic acids in high yields. Other protecting functionalities such as methyl, ethyl, tert-butyl, and trityl esters as well as benzyl ethers, tert-butyl ethers, and Nbenzylamides are unaffected under these conditions. Georg Thieme Verlag Stuttgart.
Facile Hydrolysis of Esters with KOH-Methanol at Ambient Temperature
Khurana, Jitender M.,Chauhan, Sushma,Bansal, Geeti
, p. 83 - 87 (2004)
A simple, rapid, and efficient method is reported for the hydrolysis of a variety of mono-and diesters of aromatic, aliphatic, fatty, and heterocyclic acids with potassium hydroxide in methanol at ambient temperature (~35°C).
Copper catalyzed oxidation of benzylic alcohols in water with H 2O2
Ahmad, Jahir Uddin,R?is?nen, Minna T.,Leskel?, Markku,Repo, Timo
, p. 180 - 187 (2012)
A straightforward, efficient and sustainable copper catalyzed method was developed for oxidation of benzylic alcohols with 30% H2O2 in water. The reaction proceeded with CuSO4 catalyst (1 mol%) at 100 °C without additional base or ligand. Primary benzylic alcohols were converted almost quantitatively to aldehydes with 70-90% selectivity, corresponding acids being the major side products. Also secondary benzylic alcohols afforded the corresponding ketones in high conversion with selectivities greater than 90%. It was demonstrated that the CuSO4 catalyst can be recycled and reused at least for three runs, even though with some loss of catalytic activity. Selectivity of the CuSO4 based catalyst system could be further increased by using 2-N-(p-fluorophenyl)- pyrrolecarbaldimine (1) as a ligand in combination with TEMPO in K 2CO3 solution. The catalyst system was individually optimized (1 mol% CuSO4, 2 mol% 1, 0.1 M K2CO3 and 5 mol% TEMPO) for a wide range of benzylic and allylic alcohols, which were quantitatively and selectively converted into the corresponding aldehydes with 3 eq. of H2O2 in 1 h.
Oxo osmium(VIII) complexes in oxidation: Crystal structures of OsO4·nmo (nmo = N-methylmorpholine N-oxide) and OsO4·nmm (nmm = N-methylmorpholine), and use of cis-[OsO4(OH)2]2- as an oxidation catalyst
Bailey, Alan J.,Bhowon, Minu G.,Griffith, William P.,Shoair, Abdel G. F.,White, Andrew J. P.,Williams, David J.
, p. 3245 - 3250 (1997)
The new complexes OsO4·nmo (nmo = N-methylmorpholine N-oxide) and OsO4·nmm (nmm = N-methylmorpholine) have been made, their crystal structures determined, and their possible involvement in the catalysed dihydroxylation of alkenes considered. The use of cis-[OsO4(OH)2]2- as a catalyst for the oxidation of alcohols, aldehydes and alkyl halides to carboxylic acids with [Fe(CN)6]3- and other co-oxidants and also for the cleavage and dihydroxylation of alkenes with [Fe(CN)6]3- has been investigated.
A Woven Supramolecular Metal-Organic Framework Comprising a Ruthenium Bis(terpyridine) Complex and Cucurbit[8]uril: Enhanced Catalytic Activity toward Alcohol Oxidation
Li, Zhan-Ting,Liu, Yi,Wang, Hui,Wang, Ze-Kun,Xu, Zi-Yue,Zhang, Dan-Wei,Zhang, Yun-Chang
, p. 1498 - 1503 (2020)
The self-assembly of a diamondoid woven supramolecular metal–organic framework wSMOF-1 has been achieved from intertwined [Ru(tpy)2]2+ (tpy=2,2′,6′,2′′-terpyridine) complex M1 and cucurbit[8]uril (CB[8]) in water, where the intermolecular dimers formed by the appended aromatic arms of M1 are encapsulated in CB[8]. wSMOF-1 exhibits ordered pore periodicity in both water and the solid state, as confirmed by a combination of 1H NMR spectroscopy, UV-vis absorption, isothermal titration calorimetry, dynamic light scattering, small angle X-ray scattering and selected area electron diffraction experiments. The woven framework has a pore aperture of 2.1 nm, which allows for the free access of both secondary and primary alcohols and tert-butyl hydroperoxide (TBHP). Compared with the control molecule [Ru(tpy)2]Cl2, the [Ru(tpy)2]2+ unit of wSMOF-1 exhibits a remarkably higher heterogeneous catalysis activity for the oxidation of alcohols by TBHP in n-hexane. For the oxidation of 1-phenylethan-1-ol, the yield of acetophenone was increased from 10 percent to 95 percent.
NHC catalyzed transformation of aromatic aldehydes to acids by carbon dioxide: An unexpected reaction
Nair, Vijay,Varghese, Vimal,Paul, Rony Rajan,Jose, Anu,Sinu,Menon, Rajeev S.
, p. 2653 - 2655 (2010)
A facile NHC-mediated reaction of aromatic aldehydes with carbon dioxide leading to carboxylic acids is described. The present protocol is mechanistically important, and it can serve as a tool for the sequestration of carbon dioxide.
Photo-tunable oxidation of toluene and its derivatives catalyzed by TBATB
Mardani, Atefeh,Kazemi, Foad,Kaboudin, Babak
, (2021)
In this report, tetrabutylammonium tribromide (TBATB) was introduced as an efficient visible light active catalyst to carry out the aerobic oxidation of toluene, its derivatives, and some of methyl arenes to benzaldehydes, benzoic acids and ketones in good to high yields. All the oxidation reactions were performed under mild conditions using oxygen as a green oxidant, a catalytic amount of TBATB under blue (460 nm), royal blue (430 nm), and violet LED (400 nm) irradiation. It was found that the reactions selectivity was significantly affected by changing the solvent (from CH3CN to EtOAc) and LED wavelength (from blue to violet). In the following, our mechanistic studies revealed that the visible light oxidation of toluenes and methyl arenes over TBATB could be following a benzyl peroxy radical intermediate.
Nitromethane in polyphosphoric acid- A new reagent for carboxyamidation and carboxylation of activated aromatic compou
Aksenov, Alexander V.,Aksenov, Nicolai A.,Nadein, Oleg N.,Aksenova, Inna V.
, p. 541 - 547 (2012)
A new method of carboxyamidation of aromatic compounds based on their reaction with nitromethane in polyphosphoric acid has been developed. Upon the hydrolysis of benzamides during the reaction mixture workup, the corresponding benzoic acids can be obtained. Taylor & Francis Group, LLC.
-
Kharasch,Fuchs
, p. 292,294 (1945)
-
Facile aerobic photooxidation of alcohols using 2-chloroanthraquinone under visible light irradiation
Shimada, Yoshiko,Hattori, Kasumi,Tada, Norihiro,Miura, Tsuyoshi,Itoh, Akichika
, p. 2684 - 2688 (2013)
A facile photooxidation of alcohols to obtain carboxylic acids and ketones using easily handled 2-chloroanthraquinone as an organocatalyst under visible light irradiation in an air atmosphere is reported. The reaction conditions are mild, such as an air atmosphere and ambient pressure and temperature. Georg Thieme Verlag Stuttgart New York.
Carbon nitride-catalyzed oxidative cleavage of carbon-carbon bond of α-hydroxy ketones with visible light and thermal radiation
Zhan, Haiying,Liu, Wenjie,Fu, Minling,Cen, Jinghe,Lin, Jingxin,Cao, Hua
, p. 184 - 189 (2013)
Mesoporous carbon nitride (mpg-C3N4) as a photocatalyst showed higher photocatalytic activities in organic synthesis. Herein we reported a mpg-C3N4-catalyzed oxidation of α-hydroxy ketones to synthesize benzoic acids with visible light. This reaction represented a green and facile route to synthesize benzoic acids for which catalytic approaches were scarce.
Metal-free oxidative cleavage of the C-C bond in α-hydroxy-β-oxophosphonates
Battula, Satyanarayana,Kumar, Atul,Ahmed, Qazi Naveed
, p. 9953 - 9956 (2015)
The potential of TBHP to promote oxidative hydroxylation of α-hydroxy-β-oxophosphonates (HOPs) through C(CO)-C bond cleavage is described. This cleavage, as depicted in the mechanism is expected through an isomer of HOP that reacts with TBHP to generate acids.
Cobalt(II)-Catalyzed Reaction of Aldehydes with Acetic Anhydride under an Oxygen Atmosphere: Scope and Mechanism
Bhatia, Beena,Punniyamurthy, T.,Iqbal, Javed
, p. 5518 - 5523 (1993)
The reaction of aldehydes with acetic anhydride in the presence of catalytic cobalt(II) chloride under an oxygen atmosphere at ambient temperature is dependent upon the reaction medium.Aliphatic aldehydes react in acetonitrile to give 1,2-diones whereas the aromatic aldehydes are acylated to yield the corresponding acylals.On the other hand, carboxylic acids are obtained from aliphatic and aromatic aldehydes by conducting the reaction in dichloroethane or benzene.Cobalt(II) chloride in acetonitrile catalyzes the conversion of aliphatic aldehydes to the correspondinganhydrides in the absence of acetic anhydride whereas aromatic aldehydes remain largely unaffected under these conditions.A preliminary mechanistic study in three different solvents (i.e. acetonitrile, dichloroethane, and DMF) has revealed that in acetonitrile and in the presence of acetic anhydride, aliphatic aldehydes behave differently than aromatic aldehydes.Some trapping experiments using methyl acrylate and stilbene have been conducted to demonstrate the occurence of an acyl cobalt and peroxyacyl cobalt intermediate during these reactions.
A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid
Wang, Lin,Neumann, Helfried,Beller, Matthias
, p. 6910 - 6914 (2018)
A new catalyst for the carboxylative synthesis of arylacetic and benzoic acids using formic acid (HCOOH) as the CO surrogate was developed. In an improvement over previous work, CO is generated in situ without the need for any additional activators. Key to success was the use of a specific system consisting of palladium acetate and 1,2-bis((tert-butyl(2-pyridinyl)phosphinyl)methyl)benzene. The generality of this method is demonstrated by the synthesis of more than 30 carboxylic acids, including non-steroidal anti-inflammatory drugs (NSAIDs), under mild conditions in good yields.
A new, highly selective synthesis of aromatic aldehydes by aerobic free-radical oxidation of benzylic alcohols, catalysed by n-hydroxyphthalimide under mild conditions. Polar and enthalpic effects
Minisci, Francesco,Punta, Carlo,Recupero, Francesco,Fontana, Francesca,Pedulli, Gian Franco
, p. 688 - 689 (2002)
A new selective synthesis of aromatic aldehydes is described, based on catalytic oxidation of benzyl alcohols with molecular oxygen at rt and atmospheric pressure.
A general method for the alkaline cleavage of enolisable ketones
Zabjek, Alenka,Petric, Andrej
, p. 6077 - 6078 (1999)
An efficient method is described for the cleavage of enolisable aryl methyl and aryl ethyl ketones using an excess of KOH in DMF at an elevated temperature. It presents a general hydrolytic method yielding aromatic carboxylic acids, and is complementary to the widely used oxidative methods for ketone cleavage.
Transition-Metal-Free carboxylation of organozinc reagents using CO 2 in DMF solvent
Kobayashi, Koji,Kondo, Yoshinori
, p. 2035 - 2037 (2009)
An efficient process for the carboxylation of functionalized organozinc reagents with CO2 under transition-metal-free conditions was developed by employing DMF solvent in the presence of LICl.
Cobalt-Catalyzed Reductive Carboxylation of Aryl Bromides with Carbon Dioxide
Hang, Wei,Yi, Yaping,Xi, Chanjuan
, p. 2337 - 2341 (2020)
Cobalt-catalyzed reductive carboxylation of aryl bromides with carbon dioxide has been developed. The reaction proceeded under one atm pressure of CO2 at 40 °C in the presence of cobalt iodide/2,2′-bipyridine catalysts and zinc dust as a reducing reagent. Various aryl bromides could be converted to the corresponding carboxylic acids in good to high yields. Preliminary mechanistic experiments ruled out intervention of intermediate organozinc species for carboxylation with CO2, thus suggesting a direct CO2 insertion into the corresponding ArCoBr species. (Figure presented.).
-
Hauser,Swamer,Ringler
, p. 4023,4025 (1948)
-
Cobalt (II) catalyzed oxidation of aldehydes to carboxylic acid with molecular oxygen
Bhatia, Beena,Iqbal, Javed
, p. 7961 - 7964 (1992)
A variety of aromatic and some aliphatic aldehydes are efficiently transformed to the corresponding carboxylic acid in presence of catalytic amount of Cobalt (II) chloride, molecular oxygen and acetic anhydride at room temperature. Phenolic aldehydes undergo acylative oxidation to give the corresponding acylated carboxylic acid.
Metal-Organic Framework Based on Heptanuclear Cu-O Clusters and Its Application as a Recyclable Photocatalyst for Stepwise Selective Catalysis
Zhou, Jie,Huang-Fu, Xu,Huang, Yang-Ying,Cao, Chu-Ning,Han, Jie,Zhao, Xiao-Li,Chen, Xu-Dong
, p. 254 - 263 (2020)
Visible-light driven photoreactions using metal-organic frameworks (MOFs) as catalysts are promising with regard to their environmental friendly features such as the use of renewable and sustainable energy of visible light and potential catalyst recyclability. To develop potential heterogeneous photocatalysts, a family of three copper(II) coordination polymers bearing different Cu-O assemblies have been synthesized with the ligand 4,4-disulfo-[1,1-biphenyl]-2,2-dicarboxylate acid (H4DSDC), namely, {[Cu7(DSDC)2(OH)6(H2O)10]·xH2O}n (1), {[Cu4(DSDC)(4,4-bpy)2(OH)4]·2H2O}n (2), and {Cu2(DSDC)(phen)2(H2O)2}n (3) (4,4-bpy = 4,4-bipyridine and phen = 1,10-phenanthroline). Complex 1 represents a metal-organic framework featuring a NbO type topology constructed from the infinite linkage of heptanuclear [Cu7(μ3-OH)6(H2O)10]8+ clusters by deprotonated DSDC4- ligands, comprising one-dimensional hexagonal channels of a diameter around 11 ? that are filled with water molecules. The infinite waving {[Cu2(OH)2]2+}n ladderlike chains in complex 2 are bridged by DSDC4- and 4,4-bpy ligands into a three-dimensional framework. A two-dimensional layered structure is formed in complex 3 due to the existence of terminal phenanthroline ligands. All of the coordination polymers 1-3 are able to catalyze the visible-light driven oxidation of alcohols at mild conditions using hydrogen peroxide as an oxidant, in which complex 1 demonstrates satisfactory efficiency. Significantly for this photoreaction catalyzed by 1, the extent of oxidation over aryl primary alcohols is fully controllable with time-resolved product selectivity, giving either corresponding aldehydes or carboxylate acids in good yields. It is also remarkable that the photocatalyst could be recovered almost quantitatively on completion of the catalytic cycle without any structure change, and could be recycled for catalytic use for at least five cycles with constant efficiency. This photocatalyst with time-resolved selectivity for different products may provide new insight into the design and development of novel catalytic systems.
Palladium-catalyzed oxidative coupling of arylboronic acid with isocyanide to form aromatic carboxylic acids
Chen, Zhen-Bang,Liu, Kui,Zhang, Fang-Ling,Yuan, Qing,Zhu, Yong-Ming
, p. 8078 - 8083 (2017)
A valuable palladium-catalyzed oxidative coupling of aryl- and alkenyl borides with isocyanide for the synthesis of corresponding carboxylic acids has been developed. With wide substrate scopes and good functional group tolerance, this reaction offers corresponding carboxylic acids in moderate to excellent yields.
Oxidation of Primary Alcohols and Aldehydes to Carboxylic Acids via Hydrogen Atom Transfer
Tan, Wen-Yun,Lu, Yi,Zhao, Jing-Feng,Chen, Wen,Zhang, Hongbin
, p. 6648 - 6653 (2021)
The oxidation of primary alcohols and aldehydes to the corresponding carboxylic acids is a fundamental reaction in organic synthesis. In this paper, we report a new chemoselective process for the oxidation of primary alcohols and aldehydes. This metal-free reaction features a new oxidant, an easy to handle procedure, high isolated yields, and good to excellent functional group tolerance even in the presence of vulnerable secondary alcohols and tert-butanesulfinamides.
Hydrogen Peroxide Oxidation of α-(N,N-Dialkyl)aminoketones
Wenkert, David,Eliasson, K. Margareta,Rudisill, Duane
, p. 392 - 393 (1983)
α-(N,N-Dialkyl)aminoketones are fragmented oxidatively by hydrogen peroxide, leading to carboxylic acids and products derived from iminium intermediates.
One-Pot Biocatalytic In Vivo Methylation-Hydroamination of Bioderived Lignin Monomers to Generate a Key Precursor to L-DOPA
Birmingham, William R.,Galman, James L.,Parmeggiani, Fabio,Seibt, Lisa,Turner, Nicholas J.
, (2022/01/13)
Electron-rich phenolic substrates can be derived from the depolymerisation of lignin feedstocks. Direct biotransformations of the hydroxycinnamic acid monomers obtained can be exploited to produce high-value chemicals, such as α-amino acids, however the reaction is often hampered by the chemical autooxidation in alkaline or harsh reaction media. Regioselective O-methyltransferases (OMTs) are ubiquitous enzymes in natural secondary metabolic pathways utilising an expensive co-substrate S-adenosyl-l-methionine (SAM) as the methylating reagent altering the physicochemical properties of the hydroxycinnamic acids. In this study, we engineered an OMT to accept a variety of electron-rich phenolic substrates, modified a commercial E. coli strain BL21 (DE3) to regenerate SAM in vivo, and combined it with an engineered ammonia lyase to partake in a one-pot, two whole cell enzyme cascade to produce the l-DOPA precursor l-veratrylglycine from lignin-derived ferulic acid.
Mechanochemical Grignard Reactions with Gaseous CO2 and Sodium Methyl Carbonate**
Pfennig, Victoria S.,Villella, Romina C.,Nikodemus, Julia,Bolm, Carsten
supporting information, (2022/01/22)
A one-pot, three-step protocol for the preparation of Grignard reagents from organobromides in a ball mill and their subsequent reactions with gaseous carbon dioxide (CO2) or sodium methyl carbonate providing aryl and alkyl carboxylic acids in up to 82 % yield is reported. Noteworthy are the short reaction times and the significantly reduced solvent amounts [2.0 equiv. for liquid assisted grinding (LAG) conditions]. Unexpectedly, aryl bromides with methoxy substituents lead to symmetric ketones as major products.
A Mild Heteroatom (O -, N -, and S -) Methylation Protocol Using Trimethyl Phosphate (TMP)-Ca(OH) 2Combination
Tang, Yu,Yu, Biao
, (2022/03/27)
A mild heteroatom methylation protocol using trimethyl phosphate (TMP)-Ca(OH)2combination has been developed, which proceeds in DMF, or water, or under neat conditions, at 80 °C or at room temperature. A series of O-, N-, and S-nucleophiles, including phenols, sulfonamides, N-heterocycles, such as 9H-carbazole, indole derivatives, and 1,8-naphthalimide, and aryl/alkyl thiols, are suitable substrates for this protocol. The high efficiency, operational simplicity, scalability, cost-efficiency, and environmentally friendly nature of this protocol make it an attractive alternative to the conventional base-promoted heteroatom methylation procedures.
Transformation of Thioacids into Carboxylic Acids via a Visible-Light-Promoted Atomic Substitution Process
Fu, Qiang,Liang, Fu-Shun,Lou, Da-Wei,Pan, Gao-Feng,Wang, Rui,Wu, Min,Xie, Kai-Jun
supporting information, p. 2020 - 2024 (2022/03/31)
A visible-light-promoted atomic substitution reaction for transforming thiocacids into carboxylic acids with dimethyl sulfoxide (DMSO) as the oxygen source has been developed, affording various alkyl and aryl carboxylic acids in over 90% yields. The atomic substitution process proceeds smoothly through the photochemical reactivity of the formed hydrogen-bonding adduct between thioacids and DMSO. A DMSO-involved proton-coupled electron transfer (PCET) and the simultaneous generation of thiyl and hydroxyl radicals are proposed to be key steps for realizing the transformation.
100-09-4 Process route
-
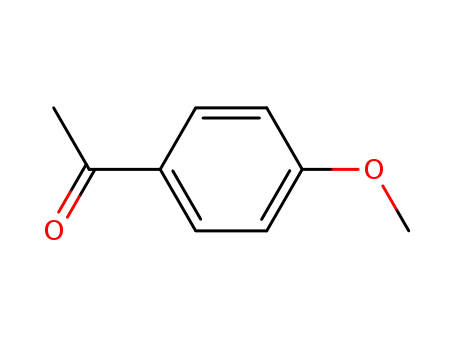
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
copper(II) nitrate trihydrate; oxygen;
In
acetonitrile;
at 120 ℃;
for 10h;
under 4500.45 Torr;
Autoclave;
|
99% |
|
With
oxygen; copper(II) nitrate;
In
acetonitrile;
at 120 ℃;
for 10h;
under 4500.45 Torr;
|
99% |
|
With
copper(l) iodide; hydroxylamine hydrochloride; oxygen;
In
dimethyl sulfoxide;
at 100 ℃;
for 8h;
Solvent;
Reagent/catalyst;
Temperature;
|
95% |
|
With
1,10-Phenanthroline; oxygen; copper diacetate; potassium hydroxide;
In
dimethyl sulfoxide;
at 130 ℃;
for 12h;
under 3000.3 Torr;
Autoclave;
|
95% |
|
1-(4-methoxyphenyl)ethanone;
With
iodine; dimethyl sulfoxide;
In
chlorobenzene;
at 130 ℃;
for 3h;
With
tert.-butylhydroperoxide;
In
chlorobenzene;
at 20 - 130 ℃;
for 3h;
Time;
|
94% |
|
With
hydroxylamine hydrochloride; iodine;
In
dimethyl sulfoxide;
at 100 ℃;
for 4h;
|
93% |
|
With
sodium hypochlorite; lithium hypochlorite;
In
ethanol;
at 77 ℃;
for 2h;
|
92% |
|
With
Iron(III) nitrate nonahydrate; iodine; oxygen; dimethyl sulfoxide;
at 130 ℃;
for 12h;
under 750.075 Torr;
Sealed tube;
Green chemistry;
|
92% |
|
With
Iron(III) nitrate nonahydrate; iodine; oxygen;
In
dimethyl sulfoxide;
at 130 ℃;
for 12h;
Sealed tube;
|
92% |
|
With
Oxone; trifluoroacetic acid;
In
1,4-dioxane;
for 10h;
Reflux;
Green chemistry;
|
90% |
|
With
iodine; dimethyl sulfoxide; copper(II) oxide;
at 90 ℃;
for 3h;
|
88% |
|
With
carbon tetrabromide; oxygen;
In
ethyl acetate;
for 12h;
Irradiation;
|
87% |
|
1-(4-methoxyphenyl)ethanone;
With
tert.-butylhydroperoxide; sodium hydroxide; tungsten(VI) oxide;
In
water;
at 80 ℃;
for 8h;
With
hydrogenchloride;
In
water;
|
85% |
|
With
oxygen; manganese (II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate;
In
acetic acid;
at 100 ℃;
for 15h;
under 760.051 Torr;
|
84% |
|
With
sodium percarbonate; potassium tert-butylate; sphingosylphosphorylcholine;
meta-dinitrobenzene;
In
tert-butyl alcohol;
at 80 ℃;
for 5h;
|
58% |
|
With
meta-dinitrobenzene; sodium hydroxide;
In
water;
at 100 ℃;
for 2.5h;
Sealed tube;
|
56.2% |
|
With
tert.-butylhydroperoxide; rhenium(VII) oxide; acetic acid;
at 100 ℃;
for 4.5h;
|
55% |
|
1-(4-methoxyphenyl)ethanone;
With
aluminum (III) chloride; N,N-dimethyl-aniline;
In
toluene;
at 100 - 110 ℃;
for 2 - 3h;
With
hydrogenchloride; water;
at 20 ℃;
pH=1 - 2;
Product distribution / selectivity;
|
15% |
|
With
Amberlyst 15;
In
toluene;
for 32h;
Reflux;
|
10% |
|
With
sodium hydroxide; chlorine;
|
|
|
With
18-crown-6 ether; acetophenone;
In
benzene;
at 25 ℃;
for 24h;
Mechanism;
relative reactivity; further oxidative agents;
|
|
|
With
sodium hydroxide; potassium chloride; potassium hexacyanoferrate(III);
In
methanol; water;
at 30 ℃;
Kinetics;
further temperature;
|
|
|
With
manganese(II) nitrate; oxygen; cobalt(II) nitrate;
In
acetic acid;
at 100 ℃;
for 6h;
|
|
|
With
copper(II) choride dihydrate; oxygen; lithium bromide; tert-butyl alcohol;
at 130 ℃;
for 10h;
under 7500.75 Torr;
Reagent/catalyst;
Autoclave;
Green chemistry;
|
|
|
With
dihydrogen peroxide;
In
water;
at 22 - 25 ℃;
for 11688h;
|
|
|
Multi-step reaction with 2 steps
1: iodine; dimethyl sulfoxide / chlorobenzene / 120 °C
2: tert.-butylhydroperoxide / 6 h
With
tert.-butylhydroperoxide; iodine; dimethyl sulfoxide;
In
chlorobenzene;
|
-
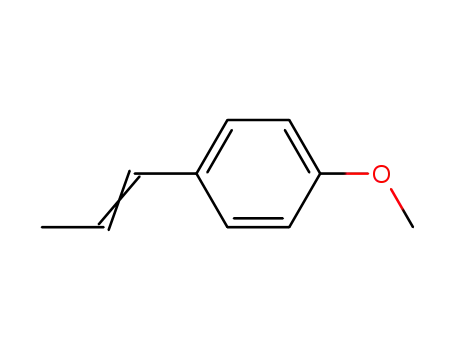
-
104-46-1,25086-72-0,63589-56-0
anethole

-
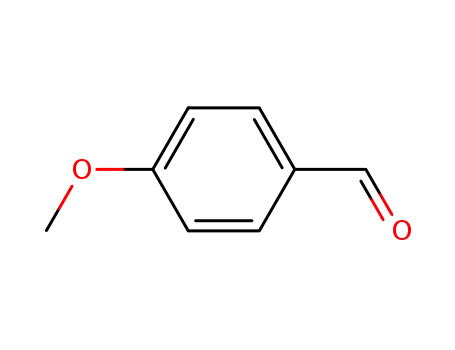
-
123-11-5
4-methoxy-benzaldehyde

-

-
100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
durch elektrolytische Oxydation in neutraler, saurer oder alkalischer waessriger Suspension;
|
100-09-4 Upstream products
-
186581-53-3

diazomethane
-
2345-34-8
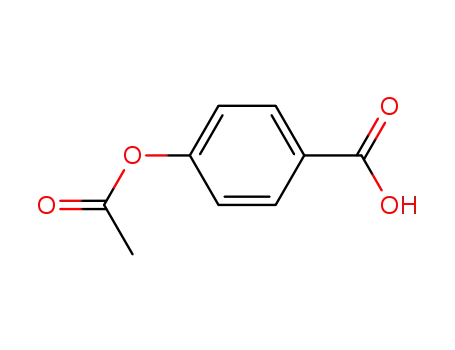
4-acetyloxy-benzoic acid
-
60-29-7
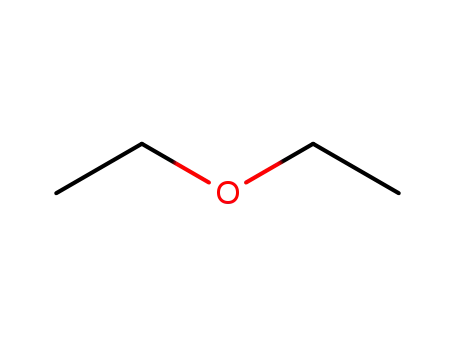
diethyl ether
-
99-96-7
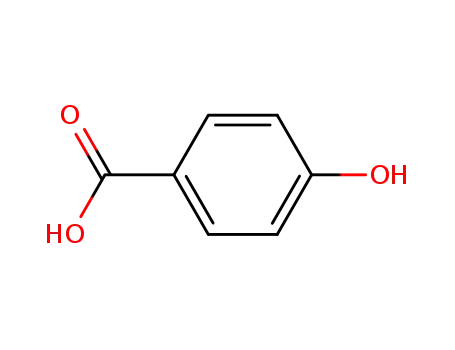
4-hydroxy-benzoic acid
100-09-4 Downstream products
-
109258-04-0
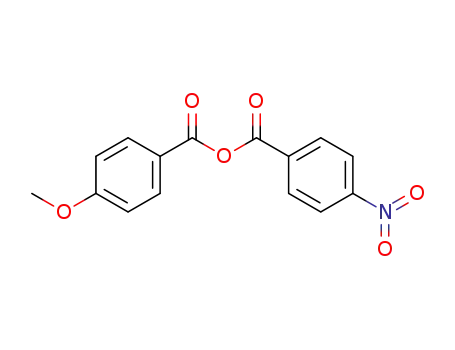
4-methoxyphenyl 4-nitrophenyl anhydride
-
37908-97-7
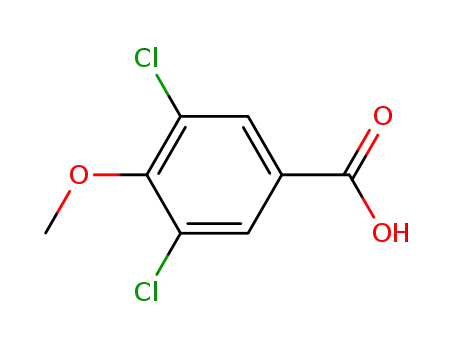
3,5-dichloro-4-methoxybenzoic acid
-
100-06-1

1-(4-methoxyphenyl)ethanone
-
121-97-1
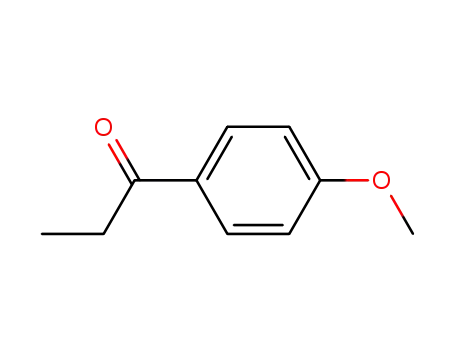
4-Methoxypropiophenone








