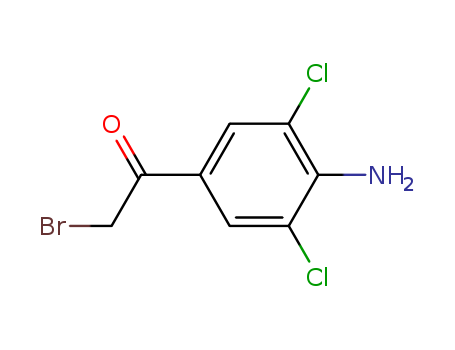
4-Amino-3,5-dichlorophenacylbromide 37148-47-3
- CasNo:37148-47-3
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
37148-47-3 Properties
- Molecular Formula:C8H6BrCl2NO
- Molecular Weight:282.952
- Appearance/Colour:Yellow solid
- Vapor Pressure:1.71E-06mmHg at 25°C
- Melting Point:142-145 °C
- Refractive Index:1.643
- Boiling Point:396.4 °C at 760 mmHg
- PKA:-2.21±0.10(Predicted)
- Flash Point:193.6 °C
- PSA:43.09000
- Density:1.764 g/cm3
- LogP:3.73440
37148-47-3 Usage
Uses
4-Amino-3,5-dichlorophenacylbromide is a compound useful in organic synthesis.
Chemical Properties
Yellow Solid
Preparation
Dissolve 4-amino-3, 5-dichloroacetophenone in chloroform and heat to 65°C. Add bromine dropwise with stirring, stir for a few minutes after adding, then add ethanol and stir. Freeze, filter out the crystals, wash with chloroform, and dry at low temperature to obtain finished products.
InChI:InChI=1/C8H6BrCl2NO/c9-3-7(13)4-1-5(10)8(12)6(11)2-4/h1-2H,3,12H2
37148-47-3 Relevant articles
Red-shifted tetra-ortho-halo-azobenzenes for photo-regulated transmembrane anion transport
Bo, Zonghua,Duarte, Fernanda,Kerckhoffs, Aidan,Langton, Matthew J.,Penty, Samuel E.
supporting information, p. 9058 - 9067 (2021/11/04)
Photo-responsive synthetic ion transporters are of interest as tools for studying transmembrane transport processes and have potential applications as targeted therapeutics, due to the possibility of spatiotemporal control and wavelength-dependent function. Here we report the synthesis of novel symmetric and non-symmetric red-shifted tetra-ortho-chloro- and tetra-ortho-fluoro azobenzenes, bearing pendant amine functionality. Functionalisation of the photo-switchable scaffolds with squaramide hydrogen bond donors enabled the preparation of a family of anion receptors, which act as photo-regulated transmembrane chloride transporters in response to green or red light. The subtle effects of chlorine/fluorine substitution,meta/parapositioning of the anion receptors, and the use of more flexible linkers are explored. NMR titration experiments on the structurally diverse photo-switchable receptors reveal cooperative binding of chloride in theZ, but notEisomer, by the two squaramide binding sites. These results are supported by molecular dynamics simulations in explicit solvent and model membranes. We show that this intramolecular anion recognition leads to effective switching of transport activity in lipid bilayer membranes, in which optimalZisomer activity is achieved using a combination of fluorine substitution andpara-methylene spacer units.
Preparation method of stable isotope labeled clenproperol
-
Paragraph 0041-0045, (2021/07/09)
The invention relates to a preparation method of stable isotope labeled clenproperol, which comprises the following steps: by taking 4-amino-3,5-dichloroacetophenone (II) as a raw material, carrying out bromination reaction to prepare a compound (III), then carrying out improved Gabriel synthesis reaction, namely amination reaction with sodium diformamide to obtain a compound (IV), then carrying out hydrolysis and reduction to obtain a compound (VI), and carrying out reductive amination reaction to generate a target compound (I). The preparation method disclosed by the invention is reasonable in process design, low in raw material price, controllable in experimental process and simple and convenient to operate, various required labeled compounds such as D-labeled, 13C-labeled or D/13C double-labeled compounds can be conveniently synthesized, and the prepared target product is high in purity, can be used as an internal standard substance in the field of food safety detection, and has important practical application value.
Novel Aryl-Substituted Pyrimidones as Inhibitors of 3-Mercaptopyruvate Sulfurtransferase with Antiproliferative Efficacy in Colon Cancer
Bantzi, Marina,Augsburger, Fiona,Loup, Jérémie,Berset, Yan,Vasilakaki, Sofia,Myrianthopoulos, Vassilios,Mikros, Emmanuel,Szabo, Csaba,Bochet, Christian G.
, p. 6221 - 6240 (2021/05/06)
The enzyme 3-mercaptopyruvate sulfurtransferase (3-MST) is one of the more recently identified mammalian sources of H2S. A recent study identified several novel 3-MST inhibitors with micromolar potency. Among those, (2-[(4-hydroxy-6-methylpyrimidin-2-yl)sulfanyl]-1-(naphthalen-1-yl)ethan-1-one) or HMPSNE was found to be the most potent and selective. We now took the central core of this compound and modified the pyrimidone and the arylketone sides independently. A 63-compound library was synthesized; compounds were tested for H2S generation from recombinant 3-MST in vitro. Active compounds were subsequently tested to elucidate their potency and selectivity. Computer modeling studies have delineated some of the key structural features necessary for binding to the 3-MST's active site. Six novel 3-MST inhibitors were tested in cell-based assays: they exerted inhibitory effects in murine MC38 and CT26 colon cancer cell proliferation; the antiproliferative effect of the compound with the highest potency and best cell-based activity (1b) was also confirmed on the growth of MC38 tumors in mice.
Phenylethanolamine β receptor agonist synthetic method
-
, (2019/07/04)
The invention discloses a phenylethanolamine β receptor agonist synthetic method, comprises the following steps: S1: the 4 - amino acetophenone dissolved in an organic solvent, with the electrophilic reagent occurs on the benzene ring substituted halogenated reaction, generating [...] intermediate; [...] intermediates in organic solvent or in water, under the catalysis of the metal catalyst with the cyanide reagent undergo nucleophilic substitution reaction, generating phenyl ketone intermediate; S2: phenyl ketone intermediates in organic solvent, with the copper bromide generating carbonyl α bromo reaction to produce α - bromoacetophenone intermediates; S3: α - bromoacetophenone intermediates in organic solvent with tert-butyl amine or isopropylamine reaction intermediates acetophenone amines; S4: acetophenone amine intermediates in organic solvent, with the reduction hydrogenation reagent react to generate the phenylethanolamine β receptor agonists; synthetic method of this invention a simple and highly efficient and cheap and easy to obtain, atom utilization is high, the synthetic product chemical purity is greater than 99%, to meet the detection requirements of the food safety.
37148-47-3 Process route
-
-
C8H5Br2Cl2NO

-
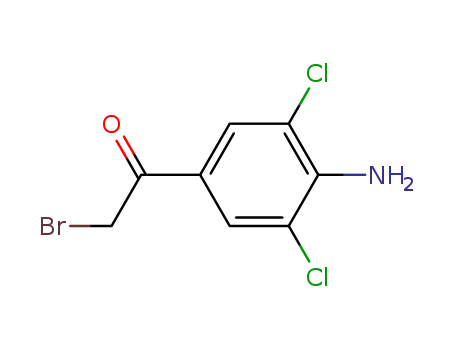
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one
| Conditions | Yield |
|---|---|
|
With
triethylamine; phosphonic acid diethyl ester;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 3h;
|
4.25 g |
-
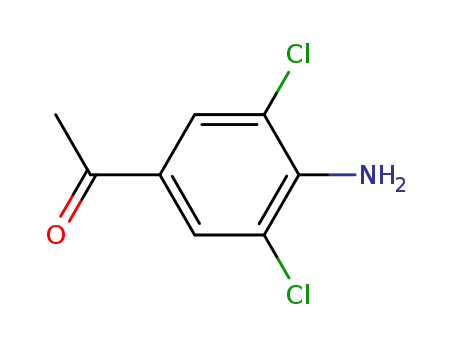
-
37148-48-4
1-(4-amino-3,5-dichlorophenyl)-1-ethanone

-

-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
C8H5Br2Cl2NO
| Conditions | Yield |
|---|---|
|
With
bromine;
In
chloroform;
for 0.25h;
Reflux;
|
42% 28% |
37148-47-3 Upstream products
-
37148-48-4

1-(4-amino-3,5-dichlorophenyl)-1-ethanone
-
608-31-1
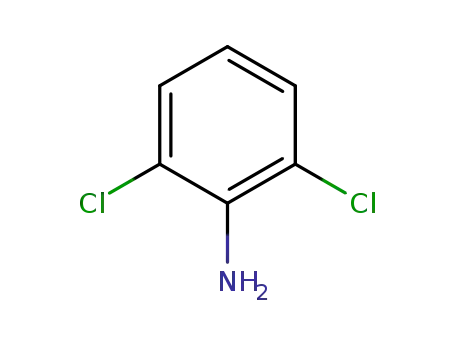
2,6-Dichloroaniline
-
99-92-3
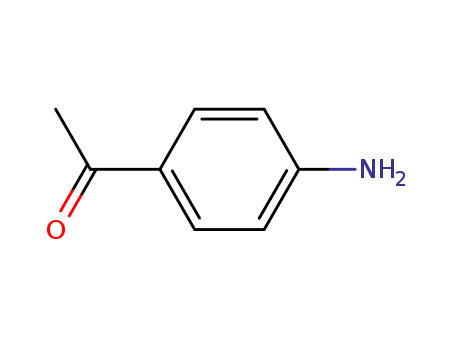
p-aminobenzophenone
37148-47-3 Downstream products
-
37163-03-4
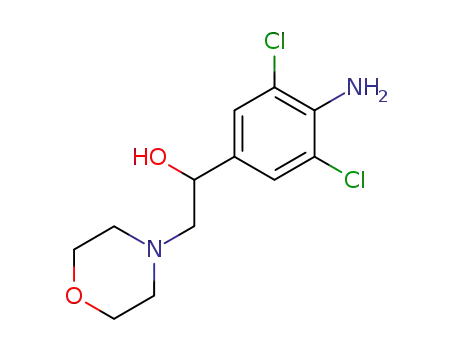
1-(4-amino-3,5-dichloro-phenyl)-2-morpholin-4-yl-ethanol
-
102198-32-3
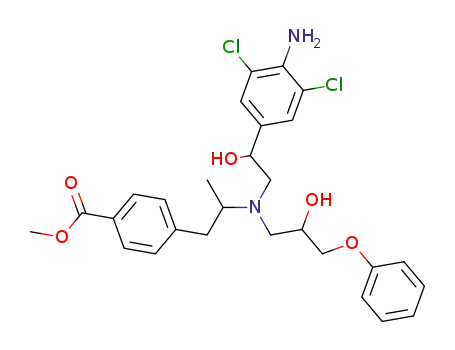
Methyl 4-[(R)-2-(N-[2-[4-amino-3,5-dichlorophenyl]-2-hydroxyethyl]-N-[(S)-2-hydroxy-3-phenoxypropyl]amino] propyl]benzoate
-
1078715-65-7
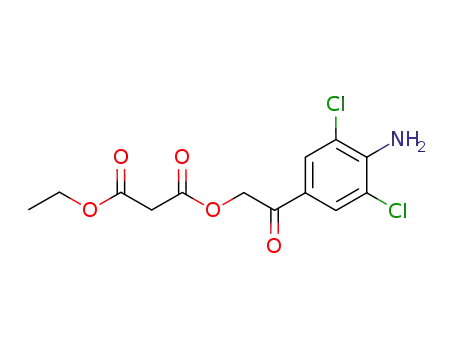
C13H13Cl2NO5
-
1078715-64-6
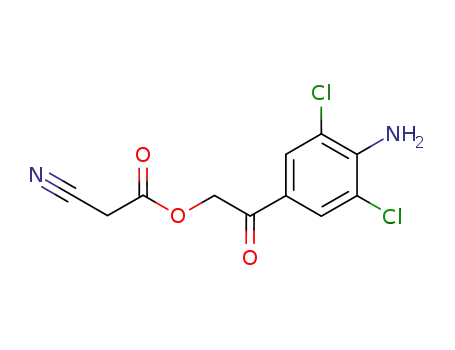
C11H8Cl2N2O3








