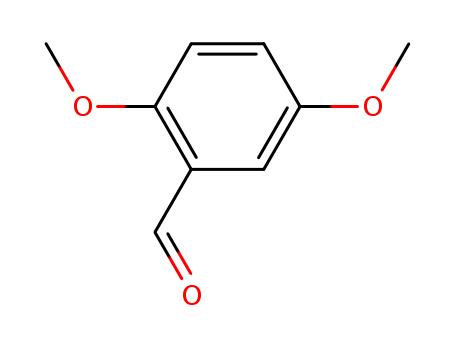
2,5-Dimethoxybenzaldehyde 93-02-7
- CasNo:93-02-7
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
93-02-7 Properties
- Molecular Formula:C9H10O3
- Molecular Weight:166.177
- Appearance/Colour:yellow crystalline solid
- Melting Point:46-48 °C(lit.)
- Refractive Index:1.5260 (estimate)
- Boiling Point:283.842 °C at 760 mmHg
- Flash Point:120.644 °C
- PSA:35.53000
- Density:1.114 g/cm3
- LogP:1.51630
93-02-7 Usage
Chemical Properties
yellow crystalline solid
Uses
2,5-Dimethoxybenzaldehyde is used in the preparation of 2,5-dimethoxyphenethylamine, which is utilized to prepare psychoactive drugs such as 2,5-dimethoxy-4-bromophenethylamine, 2,5-dimethoxy-4-iodophenethylamine and 4-Chloro-2,5-dimethoxy-phenethylamine. It acts as an intermediate in organic synthesis.
Application
2,5-Dimethoxybenzaldehyde, also known as 2C-H, is an organic compound and a benzaldehyde derivative. It can be used to produce 2,5-dimethoxyphenethylamine. 2C-H is also used to produce many other substituted phenethylamines such as 2C-B, 2C-I and 2C-C.
Preparation
2,5-Dimethoxybenzaldehyde synthesis: Anethole is oxidized to anisaldehyde , which after isolation is subjected to a BaeyerVilliger oxidation reaction with performic or peracetic acid. The O-formyl-4-methoxyphenol obtained this way is hydrolyzed . 4-Methoxyphenol is subsequently formylated using the Reimer-Tiemann method and the obtained 2-hydroxy-5-methoxybenzaldehyde is methylated with dimethylsulfate to 2,5-dimethoxybenzaldehyde.ReactionA : Anisaldehyde from anethole via oxidative cleavage: 20 g anise oil was suspended in a mixture of 150 mL water and 30 mL conc. sulfuric acid; addition of 55 g sodium bichromate at such a rate that the temperature did not exceed 40°C. The reaction mixture was extracted with 4 x 125 mL toluene and the solvent evaporated. The residual oil was vacuum distilled to yield 9.1 g anisaldehyde.B : O-formyl-4-methoxyphenol: 6 mL anisaldehyde was dissolved in 75 mL dichloromethane (DCM). A mixture of 12 g hydrogen peroxide and 10 mL conc. formic acid was added over 30 min. The reaction mixture was gently refluxed for 21 h.C: B 4-methoxyphenol: Evaporating the solvent from reaction mixture and taking up the residue in 100 mL aqueous NaOH (20%) (25 mL MeOH as co-solvent) yielded 4.1 g 4-methoxyphenol as a white crystalline product after the usual work-up and purification steps.D : Reimer-Tiemann formylation of 4-methoxyphenol: 124.1 g 4-methoxyphenol was dissolved in NaOH solution (320 g NaOH in 400 mL water). In total, 161 mL chloroform was added. The usual work-up and steam distillation yielded 109.8 g of a clear yellow oil that did not solidify upon standing at room temperature (GC/MS: 94% 2-hydroxy-5-methoxybenzaldehyde).E: D Methylation of 2-hydroxy-5-methoxybenzaldehyde: The yellow oil from was used without further purification. A 250 mL RB flask was charged with 100 mL acetone, 14 g anhydrous potassium carbonate and 10 g 2-hydroxy-5-methoxybenzaldehyde; the mixture was brought at reflux temperature and 11 g dimethyl sulfate was added. The reaction was continued for 4 hours. The solvent is evaporated and the crude end product crystallized in cold water. Recrystallization from EtOH/water yielded 8.3 g 2,5-dimethoxybenzaldehyde (GC/MS: 98%+ 2,5-dimethoxybenzaldehyde)
InChI:InChI=1/C9H10O3/c1-11-8-3-4-9(12-2)7(5-8)6-10/h3-6H,1-2H3
93-02-7 Relevant articles
Decomposition of methoxamine in aqueous solution: identification of the decomposition products.
Millard,Priaulx,Shotton
, p. 369 - 373 (1971)
-
Mechanism of Pd(OAc)2/DMSO-catalyzed aerobic alcohol oxidation: Mass-transfer-limitation effects and catalyst decomposition pathways
Steinhoff, Bradley A.,Stahl, Shannon S.
, p. 4348 - 4355 (2006)
Pd(OAc)2 in DMSO is an effective catalyst for the aerobic oxidation of alcohols and numerous other organic substrates. Kinetic studies of the catalytic oxidation of primary and secondary benzylic alcohol substrates provide fundamental insights into the catalytic mechanism. In contrast to the conclusion reached in our earlier study (J. Am. Chem. Soc. 2002, 124, 766-767), we find that Pd(II)-mediated alcohol oxidation is the turnover-limiting step of the catalytic reaction. At elevated catalyst loading, however, the rate of catalytic turnover is limited by the dissolution of oxygen gas into solution. This mass-transfer rate is measured directly by using gas-uptake methods, and it correlates with the maximum rate observed during catalysis. Initial-rate studies were complemented by kinetic analysis of the full-reaction timecourses at different catalyst concentrations. Kinetic fits of these traces reveal the presence of unimolecular and bimolecular catalyst decomposition pathways that compete with productive catalytic turnover.
Preparation method of 2,5-dimethoxyphenylacetic acid
-
Paragraph 0024-0026; 0032-0034; 0040-0042; 0047-0049; 0055, (2021/02/06)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2,5-dimethoxyphenylacetic acid, wherein the method comprises the following steps: A,reacting 1,4-dimethoxybenzene in a formylation system to obtain 2,5-dimethoxybenzaldehyde; B, reacting the 2,5-dimethoxybenzaldehyde obtained in the step A with a reducing agent, extracting a reaction system, then combining organic phases, drying, concentrating under reduced pressure and distilling a crude product to obtain 2,5-dimethoxybenzyl alcohol; C, reacting the 2,5-dimethoxybenzyl alcoholobtained in the step B with a bromination reagent to obtain 2-bromomethyl-1,4-dimethoxybenzene; and D, reacting the 2-bromomethyl-1,4-dimethoxybenzene obtained in the step C with magnesium or butyl lithium and carbon dioxide in a solvent to obtain the 2,5-dimethoxyphenylacetic acid. The yield and the total yield of the 2,5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of 2,5-dimethoxyphenylacetic acid synthesized by a Willegerdt-Kindler method.
Soluble/MOF-Supported Palladium Single Atoms Catalyze the Ligand-, Additive-, and Solvent-Free Aerobic Oxidation of Benzyl Alcohols to Benzoic Acids
Tiburcio, Estefanía,Greco, Rossella,Mon, Marta,Ballesteros-Soberanas, Jordi,Ferrando-Soria, Jesús,López-Haro, Miguel,Hernández-Garrido, Juan Carlos,Oliver-Meseguer, Judit,Marini, Carlo,Boronat, Mercedes,Armentano, Donatella,Leyva-Pérez, Antonio,Pardo, Emilio
, p. 2581 - 2592 (2021/02/16)
Metal single-atom catalysts (SACs) promise great rewards in terms of metal atom efficiency. However, the requirement of particular conditions and supports for their synthesis, together with the need of solvents and additives for catalytic implementation, often precludes their use under industrially viable conditions. Here, we show that palladium single atoms are spontaneously formed after dissolving tiny amounts of palladium salts in neat benzyl alcohols, to catalyze their direct aerobic oxidation to benzoic acids without ligands, additives, or solvents. With this result in hand, the gram-scale preparation and stabilization of Pd SACs within the functional channels of a novel methyl-cysteine-based metal-organic framework (MOF) was accomplished, to give a robust and crystalline solid catalyst fully characterized with the help of single-crystal X-ray diffraction (SCXRD). These results illustrate the advantages of metal speciation in ligand-free homogeneous organic reactions and the translation into solid catalysts for potential industrial implementation.
SUBSTITUTED CHROMEN-4-ONE FOR THE TREATMENT AND PROPHYLAXIS OF HEPATITIS B VIRUS INFECTION
-
Page/Page column 26, (2020/07/31)
The present invention provides novel compounds having the general formula: (I) wherein R1 to R10, Gi, G2 and m are as described herein, compositions including the impounds and methods of using the compounds for the treatment of hepatitis B.
Chemoselective Cross-Coupling between Two Different and Unactivated C(aryl)-O Bonds Enabled by Chromium Catalysis
Tang, Jinghua,Liu, Liu Leo,Yang, Shangru,Cong, Xuefeng,Luo, Meiming,Zeng, Xiaoming
supporting information, p. 7715 - 7720 (2020/05/20)
We report here the first example of cross-coupling between two different and unactivated C(aryl)-O bonds with chromium catalysis. The combination of a low-cost Cr(II) salt, 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) as the ligand, and magnesium as the reductant shows high reactivity in promoting the reductive cross-coupling of aryl methyl ether derivatives with aryl esters by cleavage and coupling of two different C(aryl)-O bonds under mild conditions. The formation of active low-valent Cr species by reduction of CrCl2 with Mg can be considered, which prefers to initially activate the C(aryl)-O bond of phenyl methyl ether with the chelation help of dtbpy and an o-imine auxiliary. The subsequent consecutive reduction, second C(aryl)-O activation, and reductive elimination allow for the achievement of selective cross-coupling of C(aryl)-O/C(aryl)-O bonds.
93-02-7 Process route
-
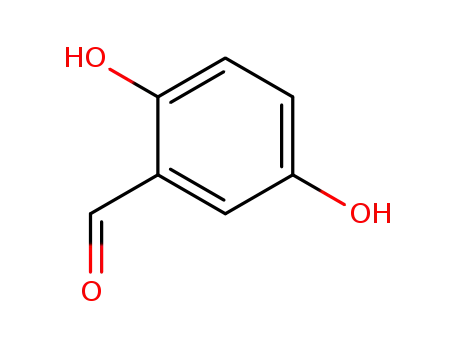
-
1194-98-5
2,5-Dihydroxybenzaldehyde

-
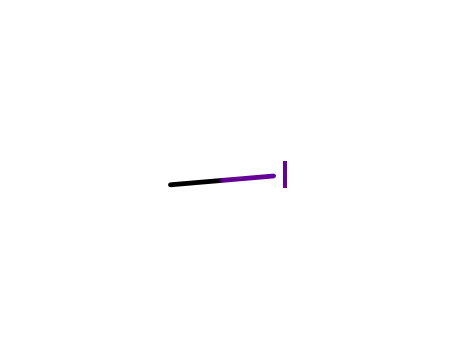
-
74-88-4
methyl iodide

-
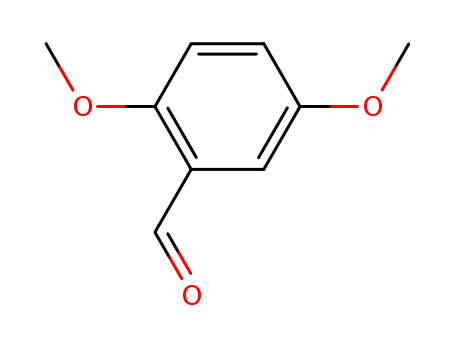
-
93-02-7
2,5-dimethoxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetone;
at 20 ℃;
for 18h;
|
85% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 1h;
|
73% |
|
With
potassium hydroxide;
|
|
|
With
potassium carbonate;
In
acetone;
at 25 ℃;
for 20h;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 16h;
|
2.4 g |
-
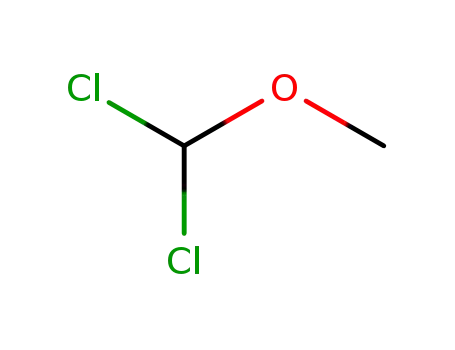
-
4885-02-3
Dichloromethyl methyl ether

-
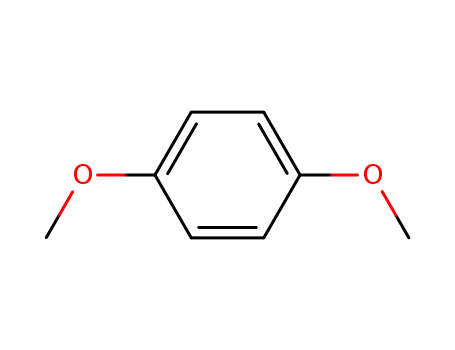
-
150-78-7
1,4-dimethoxybezene

-

-
93-02-7
2,5-dimethoxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
titanium tetrachloride;
In
dichloromethane;
at 0 ℃;
|
100% |
|
With
titanium tetrachloride;
In
dichloromethane;
at -5 - 20 ℃;
for 1.5h;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
97.6% |
|
With
titanium tetrachloride;
In
dichloromethane;
at 20 ℃;
for 0.25h;
|
93-02-7 Upstream products
-
10538-51-9
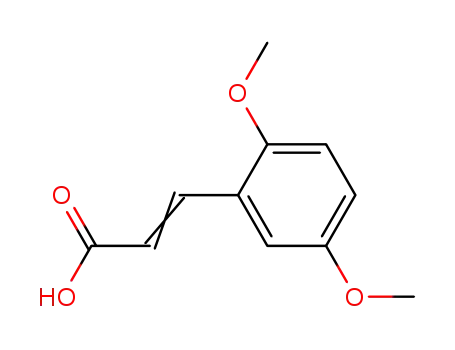
2,5-dimethoxycinnamic acid
-
17918-14-8
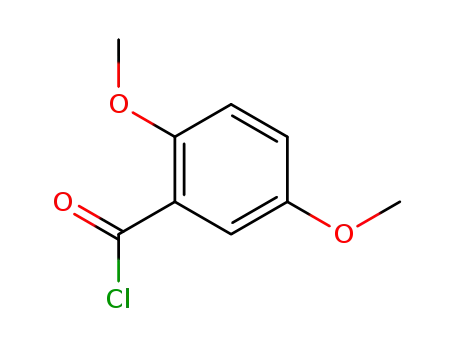
2,5-dimethoxybenzoyl chloride
-
546-67-8
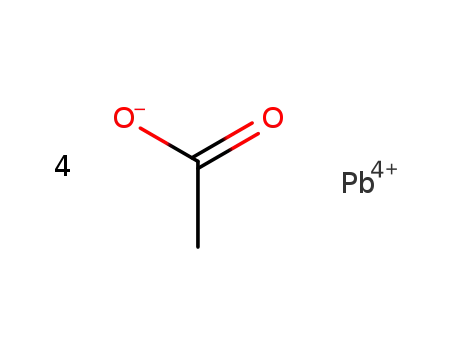
lead(IV) tetraacetate
-
64-19-7
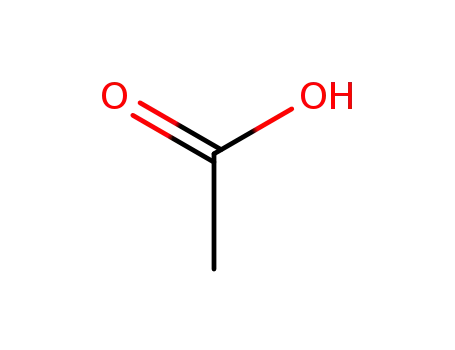
acetic acid
93-02-7 Downstream products
-
115604-02-9
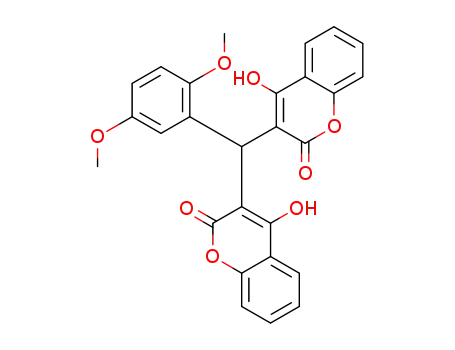
3,3'?((2,5?dimethoxy)methylene)?bis?(4?hydroxy?2H?chromene?2?one)
-
80777-95-3
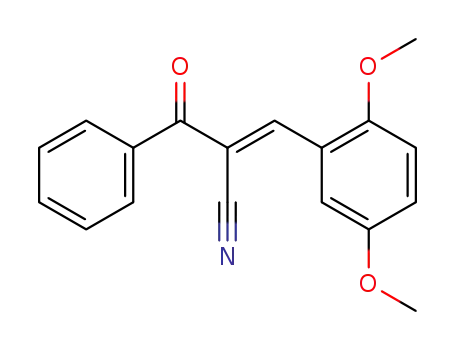
2-benzoyl-3-(2,5-dimethoxy-phenyl)-acrylonitrile
-
19785-02-5
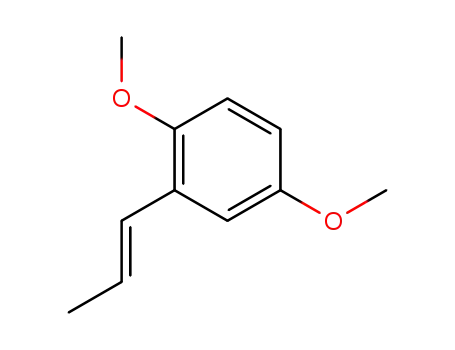
(E)-1,4-dimethoxy-2-(prop-1-enyl)benzene
-
26122-90-7
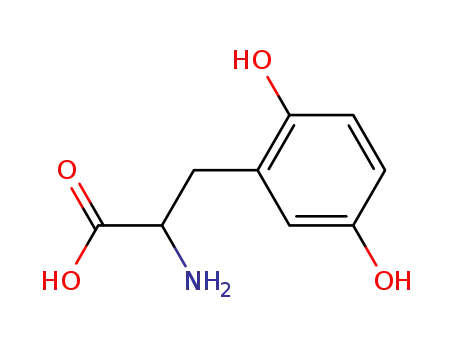
2-amino-3-(2,5-dihydroxyphenyl)propanoic acid








