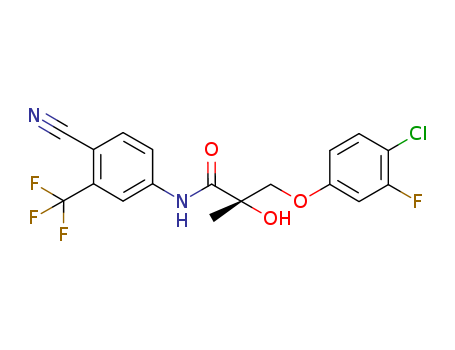
Product Details
1010396-29-8 Properties
- Molecular Formula:C18H13ClF4N2O3
- Molecular Weight:416.759
- Melting Point:119-121 °C
- Boiling Point:577.1±50.0 °C(Predicted)
- PKA:12.04±0.29(Predicted)
- PSA:82.35000
- Density:1.48±0.1 g/cm3(Predicted)
- LogP:4.21108
1010396-29-8 Usage
Description
S-23, the orally bioavailable nonsteroidal SARM, is appreciated by jocks over the world for its one of a kind capacity to improve fit bulk and bone tissue. A more secure option in contrast to anabolic steroid, this particular androgen receptor modulator is incredibly powerful to lose obstinate fat. Considered by numerous individuals as an amazing form of S-4 (Andarine), S-23 is a standout amongst other execution upgrading medications to solidify muscle and increase a grainier stylish look.
Uses
S 23 is a selective androgen receptor modulator.
benefits
Increments both skeletal and bulkAdvances durable bulk and perseverance gainsPerfect for muscle solidifyingTotally sets off catabolism in a calorie shortageBuilds bone quality and decreases fat massImproves drive in peopleEncourages clients to hold increases long after a cycleNo swelling or liquid maintenanceStep by step instructions to Use S-23
Biological Activity
S-23 is a selective androgen receptor modulator (SARM). It binds to the AR (Ki = 1.7 nM) and induces AR-mediated transcriptional activation in CV-1 cells expressing the human receptor when used at a concentration of 10 nM. S-23 increases prostate, seminal vesicle, and levator ani muscle weights in castrated rats. It decreases testicular sperm concentration without reducing mounting behavior or the number of intromissions in intact rats when administered in combination with estradiol benzoate (Item No. 10006487). S-23 (3 mg/kg) also increases preference for sexually active intact males when administered to ovariectomized female rats.
Clinical Use
S-23 is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc as a potential male hormonal contraceptive. It binds to the androgen receptor more strongly than older drugs such as andarine with a Ki of 1.7 nM, and in animal studies it showed both a good ratio of anabolic to androgenic effects, and dose-dependent suppression of spermatogenesis with spontaneous recovery after cessation of treatment.
1010396-29-8 Relevant articles
METHODS OF TREATING UROLOGICAL DISORDERS USING SARMs
-
Paragraph 0450-0451, (2016/05/10)
The present invention is directed to methods of treating, preventing, suppressing and/or inhibiting urological disorders such as urinary incontinence including stress urinary incontinence and pelvic-floor disorders by administering a SARM compound of the invention.
A METHOD OF TREATING ANDROGEN RECEPTOR (AR)-POSITIVE BREAST CANCERS WITH SELECTIVE ANDROGEN RECEPTOR MODULATOR (SARMS)
-
Paragraph 000320-000321, (2014/02/15)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and/or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; 1) prolonging survival of a subject with breast cancer, and/or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Effect of B-ring substitution pattern on binding mode of propionamide selective androgen receptor modulators
Bohl, Casey E.,Wu, Zengru,Chen, Jiyun,Mohler, Michael L.,Yang, Jun,Hwang, Dong Jin,Mustafa, Suni,Miller, Duane D.,Bell, Charles E.,Dalton, James T.
supporting information; experimental part, p. 5567 - 5570 (2009/06/30)
Selective androgen receptor modulators (SARMs) are essentially prostate sparing androgens, which provide therapeutic potential in osteoporosis, male hormone replacement, and muscle wasting. Herein we report crystal structures of the androgen receptor (AR) ligand-binding domain (LBD) complexed to a series of potent synthetic nonsteroidal SARMs with a substituted pendant arene referred to as the B-ring. We found that hydrophilic B-ring para-substituted analogs exhibit an additional region of hydrogen bonding not seen with steroidal compounds and that multiple halogen substitutions affect the B-ring conformation and aromatic interactions with Trp741. This information elucidates interactions important for high AR binding affinity and provides new insight for structure-based drug design.
1010396-29-8 Process route
-
-
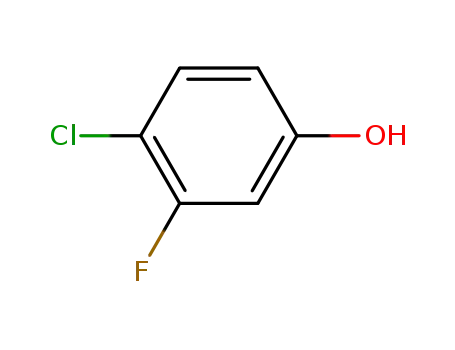
348-60-7
4-chloro-3-fluorophenol

-
![(2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide](/upload/2023/2/edff9da7-23c4-4272-b9c3-ddead02d1311.png)
-
206193-17-1
(2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide

-
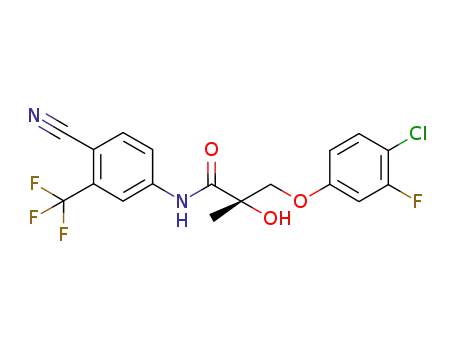
-
1010396-29-8
(S)-3-(4-chloro-3-fluorophenoxy)-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methylpropanamide
| Conditions | Yield |
|---|---|
|
(2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide;
With
potassium carbonate;
for 2h;
Reflux;
4-chloro-3-fluorophenol;
With
potassium carbonate;
In
isopropyl alcohol;
for 3h;
Reflux;
|
70.5% |
|
With
potassium carbonate;
In
isopropyl alcohol;
for 3h;
Reflux;
|
70.5% |
-
![(2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamide](/upload/2023/2/910d9d32-7dd8-4d1e-8a81-e1a84ff47839.png)
-
512776-95-3
(2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamide

-

-
348-60-7
4-chloro-3-fluorophenol

-

-
1010396-29-8
(S)-3-(4-chloro-3-fluorophenoxy)-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methylpropanamide
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
butanone;
Reflux;
|
1010396-29-8 Upstream products
-
512776-95-3
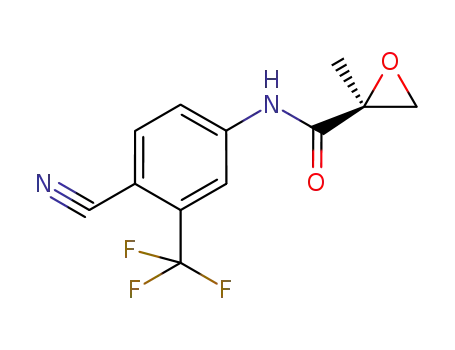
(2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyloxirane-2-carboxamide
-
348-60-7

4-chloro-3-fluorophenol
-
206193-17-1
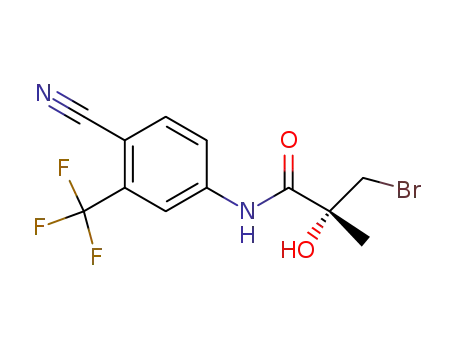
(2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide
-
654-70-6
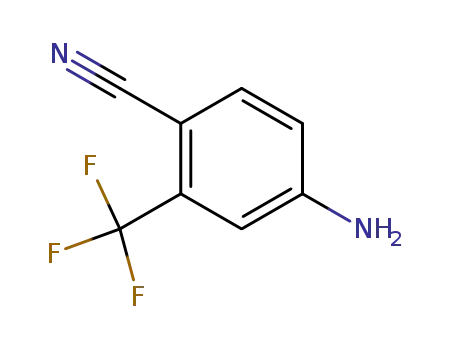
4-amino-2-trifluoromethylbenzonitrile








