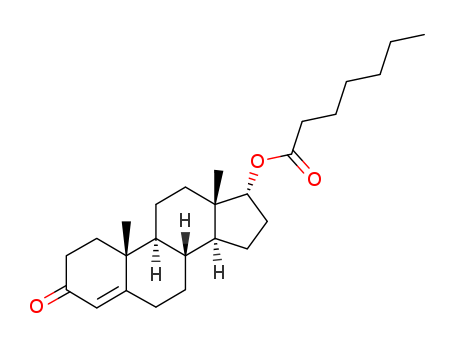
Product Details
315-37-7 Properties
- Molecular Formula:C26H40O3
- Molecular Weight:400.602
- Appearance/Colour:White crystalline powder
- Melting Point:34-39 °C
- Refractive Index:1.4700 (estimate)
- Boiling Point:503.9 °C at 760 mmHg
- Flash Point:214.2 °C
- PSA:43.37000
- Density:1.06 g/cm3
- LogP:6.40050
315-37-7 Usage
Indications and Usage
Testosterone enanthate is a type of androgen drug, and it is a synthetic compound of testosterone proprionate and testosterone enantate. Its effects are the same as those of testosterone acetate, which include promoting the development of male sex organs and secondary sex characteristics and resisting estrogen. It also has protein assimilating effects, which allow it to help muscle growth and weight gain. Testosterone enanthate can inhibit the pituitary gland and promote sex hormone secretion, thus decreasing the endogenous testosterone secreted by Leydig cells, which achieves the purpose of stopping spermatogenesis. Testosterone enanthate can be used as a long-term male birth control drug. It is clinically used to treat male hypogonadism and gonadal insufficiency, sex organ hypogenesis, infertility, eunuchism, and cryptorchidism. It can also be used to treat female dysfunctional uterine bleeding, menopausal syndrome, breast cancer and uterine cancer. It can also treat cirrhosis, aplastic anemia, osteoporosis and other consumptive diseases.
Drug Metabolism
After injection, it creates a blood concentration higher than the physiological level, and then blood concentration decreases rapidly.
Clinical Research
In a trial that injected monkeys with testosterone enanthate every week, the monkeys’ sperm cells began experiencing apoptosis in a week, with apoptosis rates peaking in the fifth week. A clinical trial with 308 participants also showed that testosterone enanthate’s birth-control effects are much more effective on Asian men than on Caucasian men, as over 95% of Asian men achieved azoospermia, while only 40-70% of Caucasian men did. A trial of over 100 volunteers showed that weekly muscle injections of 250mg led to most men developing azoospermia or severe ogliospermia in 10 weeks, and that sperm count could recover after ceasing injection for a certain period of time. If the injection intervals are extended to over 10-12 days, even increasing dosage will not have desired effects. Testosterone enanthate only needs to be injected once a week, which is an advantage over testosterone acetate.
Adverse reactions
Large dosages or extended use can cause water and sodium retention and edema. Young women may exhibit virilism, with characteristics including deepened voice, hair growth, acne, clitoral hypertrophy, etc. Not to be used by patients with prostatic cancer or pregnant patients. Should be used with caution by patients with prostate hypertrophy and liver and kidney dysfunction. When treating breast cancer, drug usage should be stopped immediately if hypercalcemia is detected.
Description
Testosterone enanthate (Item No. 22546) is an analytical reference standard categorized as an anabolic androgenic steroid. It is an ester of the naturally occurring androgen, testosterone (Item Nos. 15645 | ISO60154) with a longer half-life. Formulations containing testosterone enanthate have been tested for use in hypogonadism and as a potential male contraceptive. Formulations containing it have been associated with higher levels of total cholesterol, low density lipoproteins, and triglycerides and lower levels of high density lipoproteins. Anabolic steroids, including testosterone enanthate, have been used to enhance physical performance in racehorses and athletes, and methods to detect steroids, their derivatives, and their metabolites have been developed. This product is intended for research and forensic applications.
Chemical Properties
White or yellowish-white, crystalline powder.
Originator
Delatestryl,Squibb,US,1954
Uses
Testosterone Enanthate is a derivative of testosterone (T155000), the principal hormone of the testes, produced by the interstitial cells.
Definition
ChEBI: Testosterone enanthate is a heptanoate ester and a sterol ester. It has a role as an androgen. It is functionally related to a testosterone.
Manufacturing Process
A mixture of testosterone, pyridine and oenanthic acid anhydride is heated for 1 1/2 hours to 125°C. The cooled reaction mixture is decomposed with water while stirring and cooling. After prolonged standing at a temperature below room temperature, the whole is extracted with ether and the ethereal solution is washed consecutively with dilute sulfuric acid, water, 5% sodium hydroxide solution, and again with water. The crude ester remaining on evaporation of the dried ether solution, after recrystallization from pentane, melts at 36° to 37.5°C.
Therapeutic Function
Androgen
InChI:InChI=1/C19H28O.C7H14O2/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18;1-2-3-4-5-6-7(8)9/h12,15-17H,3-11H2,1-2H3;2-6H2,1H3,(H,8,9)/p-1/t15-,16-,17-,18-,19-;/m0./s1
315-37-7 Relevant articles
Preparation method of alkyl acid testosterone
-
Paragraph 0022-0023, (2020/11/10)
The invention discloses a preparation method of alkyl acid testosterone, and belongs to the technical field of medicine preparation and processing. According to the method, testosterone serves as a raw material and is esterified into testosterone ester, a solvent used in the esterification reaction is a non-water-soluble organic solvent, the amount of wastewater is reduced, the solvent can be recycled, and the process is more environmentally friendly. The method is high in yield, the total molar yield of the final product is higher than 85%, and the method has extremely high commercial competitiveness, is suitable for industrial large-scale production and has good economic benefits.
Synthesis method of alkyl acid testosterone
-
, (2020/12/10)
The invention discloses a synthesis method of alkyl acid testosterone, and belongs to the technical field of synthesis and processing of medicines. The method comprises the following steps of: taking4-androstenedione (4AD) as an initial raw material, firstly, carrying out enol ether protection on the keto group at the site 3, and reducing carbonyl at the site 17 into hydroxyl; or taking 4-androstenedione (4AD) as an initial raw material, firstly carrying out enol ether protection on the keto group at the site 3, then reducing carbonyl at the site 17 into hydroxyl, then carrying out hydrolysison the site 3 to obtain testosterone, and carrying out esterification and third-site hydrolysis to obtain the testosterone ester after testosterone third-site ketal protection. According to the method disclosed by the invention, the third site is protected during esterification reaction, the generation of impurities can be reduced, and an esterification reaction solvent is a water-insoluble organic solvent, so that after the reaction is completed, products can be directly extracted in a layered manner, a large amount of water does not need to be added to separate out the products, the amountof wastewater is reduced, the solvent can be recycled, and the process is more suitable for industrial production.
Long-range effect of 17-substituents in 3-oxo steroids on 4,5-double bond hydrogenation
Sidova, Romana,Stransky, Karel,Kasal, Alexander,Slavikova, Barbora,Kohout, Ladislav
, p. 1528 - 1542 (2007/10/03)
The long-range effect of substituents in the 17-position on the hydrogenation of double bond of the steroidal Δ4-3-ketones in acetic acid on a platinum catalyst is described in a series of testosterone (1) and epitestosterone (5) esters with carboxylic acids of varying alkyl chain length. The ratio 5α-to 5β-products is affected by the nature of substituents in the position 17.
315-37-7 Process route
-
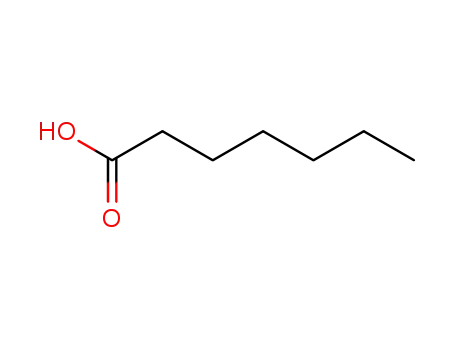
-
111-14-8
oenanthic acid

-
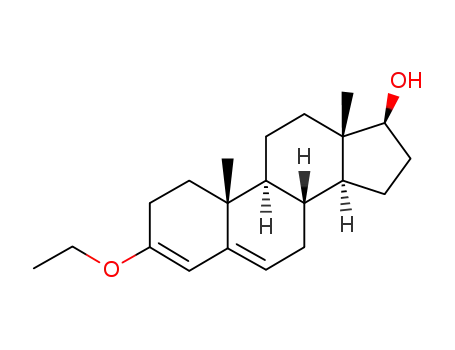
-
26614-48-2,165304-83-6
17β-hydroxy-3-ethoxyandrosta-3,5-diene

-
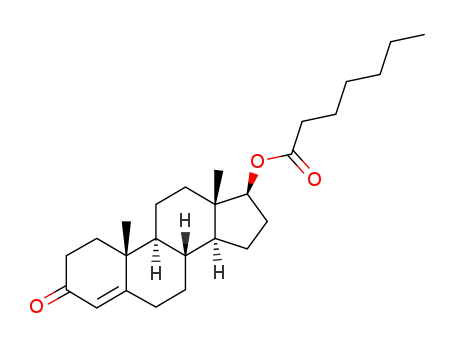
-
315-37-7
testosterone heptanoate
| Conditions | Yield |
|---|---|
|
oenanthic acid; 17β-hydroxy-3-ethoxyandrosta-3,5-diene;
With
dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
1,2-dichloro-ethane;
at 20 ℃;
Inert atmosphere;
With
sulfuric acid;
In
water;
|
96.3% |
-

-
111-14-8
oenanthic acid

-
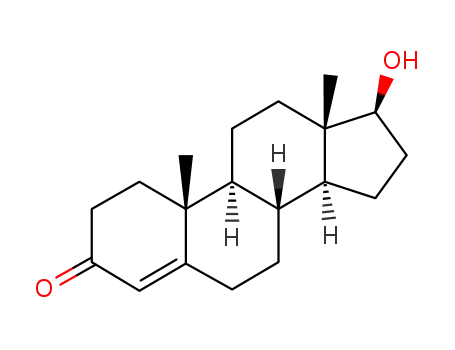
-
58-22-0
testosterone

-

-
315-37-7
testosterone heptanoate
| Conditions | Yield |
|---|---|
|
oenanthic acid; testosterone;
With
dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
1,2-dichloro-ethane;
at 20 ℃;
Inert atmosphere;
With
sulfuric acid;
In
water;
|
89.6% |
315-37-7 Upstream products
-
58-22-0

testosterone
-
2528-61-2
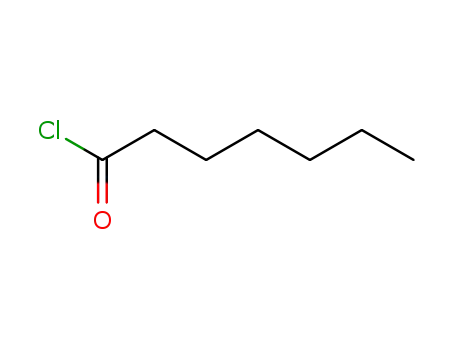
Heptanoic acid chloride
-
972-46-3
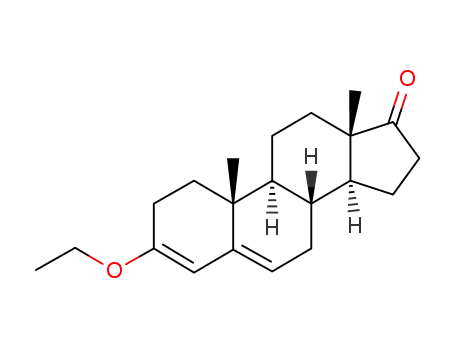
3-ethoxyandrosta-3,5-dien-17-one
-
26614-48-2

17β-hydroxy-3-ethoxyandrosta-3,5-diene
315-37-7 Downstream products
-
58-22-0

testosterone
-
63-05-8
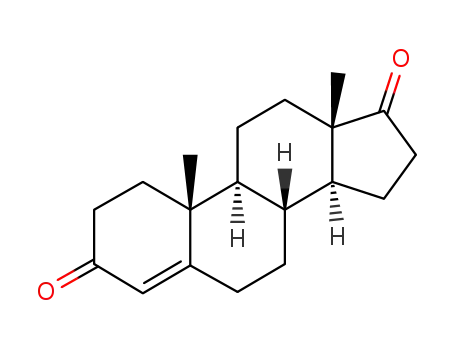
Androstenedione
-
62-99-7
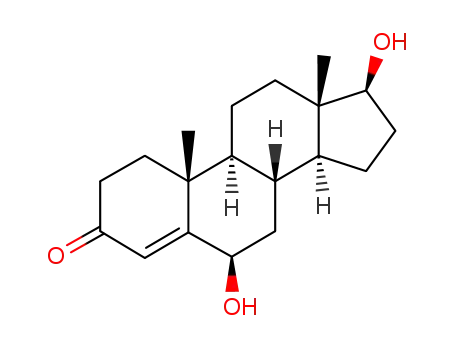
6β-hydroxytestosterone
-
4075-20-1
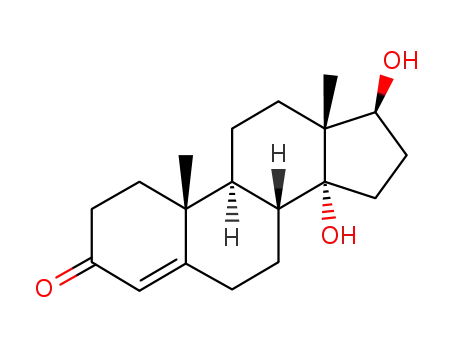
14alpha-Hydroxytestosterone