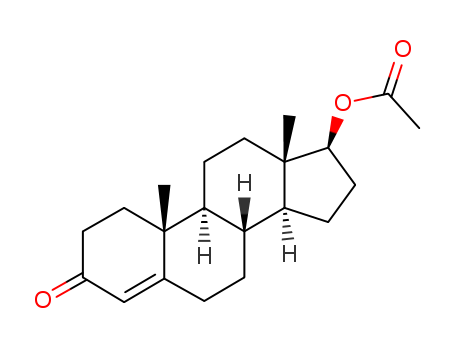
Product Details
1045-69-8 Properties
- Molecular Formula:C21H30O3
- Molecular Weight:330.467
- Appearance/Colour:white to off-white crystalline powder
- Vapor Pressure:5.28E-08mmHg at 25°C
- Melting Point:139-141 °C
- Refractive Index:1.54
- Boiling Point:441.803 °C at 760 mmHg
- Flash Point:191.472 °C
- PSA:43.37000
- Density:1.12 g/cm3
- LogP:4.45000
1045-69-8 Usage
Description
Testosterone acetate is an analytical reference standard categorized as an anabolic androgenic steroid. It is an ester of the naturally occurring androgen, testosterone. Testosterone acetate is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications.
Chemical Properties
White Solid
Uses
Testosterone Acetate is a Controlled substance that is less popular due to Acetate solution, which is aqueous solution, instead of oil solution as other esterified versions of testosterone that is offering much longer half life. It does not have any medical uses when it comes with Acetate ester. However, taken in consideration that it works in the same way as any other esterified testosterone, it can be used for the same purposes as the esterified testosterones.
Definition
ChEBI: Testosterone acetate is an androstanoid that is the acetate derivative of testosterone. It has a role as a human metabolite. It is a sterol ester, an androstanoid and a 3-oxo-Delta(4) steroid. It is functionally related to a testosterone.
General Description
Testosterone acetate is an androgen ester and an intermediate in the synthesis of testosterone from progesterone, isolated from microorganisms.
InChI:InChI=1/C21H30O3/c1-13(22)24-19-7-6-17-16-5-4-14-12-15(23)8-10-20(14,2)18(16)9-11-21(17,19)3/h12,16-19H,4-11H2,1-3H3
1045-69-8 Relevant articles
-
Bellus,D. et al.
, p. 971 - 1009 (1969)
-
An easy deoxygenation of conjugated epoxides
Righi, Giuliana,Bovicelli, Paolo,Sperandio, Anna
, p. 1733 - 1737 (2000)
An easy and high yielding transformation of epoxyketones and phenyl substituted epoxides to trans olefins in a convergent diastereoselective process is reported. The method was applied to the selective C-25 hydroxy- functionalisation of 3-keio-Δ4-cholestan-3-one, a key intermediate for the synthesis of C-25 hydroxy vitamin D3. (C) 2000 Elsevier Science Ltd.
Improved Method for the Conversion of Enol Lactones to Cyclic α,β-Unsaturated Ketones
Aristoff, Paul A.
, p. 1765 - 1766 (1985)
-
Design, synthesis and biochemical studies of new 7α-allylandrostanes as aromatase inhibitors
Varela, Carla L.,Amaral, Cristina,Correia-Da-Silva, Georgina,Carvalho, Rui A.,Teixeira, Natércia A.,Costa, Saul C.,Roleira, Fernanda M.F.,Tavares-Da-Silva, Elisiário J.
, p. 662 - 669 (2013)
Two series of derivatives of 7α-allylandrostenedione, namely its 3-deoxo and 1-ene analogs, were designed and synthesised and their biochemical activity towards aromatase evaluated. In each of these series, the C-17 carbonyl group was further replaced by the hydroxyl and acetoxyl groups. The attained data pointed out that the absence of the C-3 carbonyl group led to a slightly decrease in the inhibitory activity and the introduction of an additional double bond in C-1 revealed to be a very beneficial structural change in the studied compounds (compound 12, IC50 = 0.47 μM, Ki = 45.00 nM). Furthermore, the relevance of the C-17 carbonyl group in the D-ring as a structural feature required to achieve maximum aromatase inhibitory activity is also observed for this set of derivatives.
Synthesis and preliminary in vitro biological evaluation of 7α-testosterone-chlorambucil hybrid designed for the treatment of prostate cancer
Bastien, Dominic,Hanna, Rana,Leblanc, Valérie,Asselin, éric,Bérubé, Gervais
, p. 442 - 447 (2013)
The synthesis of 7α-testosterone-chlorambucil hybrid is reported. This compound is made from testosterone in a 6 step reaction sequence and with 23% overall yield. An alternative convergent reaction sequence yielded the same hybrid through a Grubbs metathesis reaction between chlorambucil allyl ester and 7α-allyltestosterone with 35% overall yield. MTT assays showed that the hybrid is selective towards hormone-dependent prostate cancer cell line (LNCaP (AR+)) and shows similar activity than the parent drug, chlorambucil. Thus, the new hybrid shows promising potential for drug targeting of hormone-dependent prostate cancer through its capacity of delivering chlorambucil directly to the site of treatment. This could extend the use of chlorambucil to prostate cancer in the future.
-
Dauben,W.G. et al.
, p. 4060 - 4069 (1968)
-
Synthesis and characterization of dimeric steroids based on 5-oxo-4,5-seco-yne units linked by a diyne spacer
Valdez-García, Ricardo M.,Alarcón-Manjarrez, Carlos,Arcos-Ramos, Rafael,Flores-álamo, Marcos,Iglesias-Arteaga, Martín A.
, p. 13 - 22 (2018)
New dimeric steroids in which two 5-oxo-4,5-seco-3-yne steroids units are linked by a flexible diyne spacer, were prepared by both Eglinton and Pd-catalyzed coupling of the corresponding monomers. X-Ray crystallography shows that one of the obtained dimers displays a novel supramolecular network in which the facial hydrophobicity of the steroidal skeleton plays an important role. The crystal packing is dominated by interactions that accommodate the steroid cores in a highly crowded packed columnar self-assembly. Unambiguous NMR characterization of the obtained compounds is also provided.
Desulphurization of the thioketal of testosterone acetate with deactivated Raney nickel.
Permutti,Danieli,Mazur
, p. 5425 - 5431 (1968)
-
Aspects of the progesterone response in Hortaea werneckii: Steroid detoxification, protein induction and remodelling of the cell wall
Krizancic Bombek, Lidija,Lapornik, Ajda,Ukmar, Marjeta,Matis, Maja,Cresnar, Bronislava,Katalinic, Jasna Peter,Zakelj-Mavric, Marija
, p. 1465 - 1474 (2008)
Progesterone in sublethal concentrations temporarily inhibits growth of Hortaea werneckii. This study investigates some of the compensatory mechanisms which are activated in the presence of progesterone and are most probably contributing to escape from growth inhibition. These mechanisms lead on the one hand to progesterone biotransformation/detoxification but, on the other, are suggested to increase the resistance of H. werneckii to the steroid. Biotransformation can detoxify progesterone efficiently in the early logarithmic phase, with mostly inducible steroid transforming enzymes, while progesterone biotransformation/detoxification in the late logarithmic and stationary phases of growth is not very efficient. The relative contribution of constitutive steroid transforming enzymes to progesterone biotransformation is increased in these latter phases of growth. In the presence of progesterone, activation of the cell wall integrity pathway is suggested by the overexpression of Pck2 which was detected in the stationary as well as the logarithmic phase of growth of the yeast. Progesterone treated H. werneckii cells were found to be more resistant to cell lysis than mock treated cells, indicating for the first time changes in the yeast cell wall as a result of treatment with progesterone.
TRANSFORMATION OF ANDROSTANE DERIVATIVES BY SPIRODELA OLIGORRHIZA
Tlomak, Elzbieta,Pawlowicz, Pawel,Czerwinski, Witold,Siewinski, Antoni
, p. 61 - 64 (1986)
Spirodela oligorrhiza (duckweed) of transforming some steroids of the androstane series.Hydrolysis of the acetates of testosterone and of 3-β-hydroxyandrost-5-en-17-one by this species yielded the corresponding alcohols.Further transformation of testosterone and reduction of androst-4-ene-3,17-dione indicated the interconversions of the hyroxy-ketone function on C-17 and redution of the Δ4-double bond to the trans-A/B system.Only a trace amount of 3β-hydroxyandrost-5-en-17-one underwent further transformations.
HYDROXYLATION OF PROGESTERONE BY CEPHALOSPORIUM APHIDICOLA
Farooq, Afgan,Hanson, James R.,Iqbal, Zahida
, p. 723 - 726 (1994)
The fungus, Cephalosporium aphidicola, has been shown to hydroxylate progesterone predominantly at the 6β- and 11α-positions.Minor metabolites include tetstosterone acetate, the 20(R)-alcohol and 12β,17α-dihydroxyprogesterone.The sequence involves hydroxylation at 11α and then 6β.The hydroxylations of 11α- and 17α-hydroxyprogesterone and 9β,10α-retroprogesterone have also been examined in the light of these results. - Key words: Cephalosporium aphidicola; progesterone; 9β,10α-retroprogesterone; steroid; microbiological hydroxylation.
-
Oppenauer
, (1955)
-
-
Ruzicka,Wettstein
, p. 1264,1273 (1935)
-
Highly efficient, solvent-free esterification of testosterone promoted by a recyclable polymer-supported tosylic acid catalyst under microwave irradiation
Borowiecki, Pawe?,Kraszewski, Maciej
, p. 288 - 305 (2019)
Although the classical acylation of testosterone clearly benefits from a broad substrate scope and available catalysts, the requirement of hazardous reagents and the high waste production are its drawbacks. To optimize the process efficiency as well as minimize the environmental impact, we decided to develop a novel method of testosterone esters synthesis, which relies on the usage of recyclable heterogeneous polymer-supported tosylic acid catalyst and microwave-assistance effect in a non-solvent system. Under the established MW-conditions, the acceleration of the process rate was so efficient that the reaction completed within 2.5 min, thus affording the desired esters in the 33–96% yield range without using a work-up procedure. Furthermore, the elaborated catalytic system could be recycled for at least 2 runs not only without a loss of the products yield, but unexpectedly with significant improvement of the reaction efficiency, which may indicate that the reduction of the catalyst loading is possible. We believe that this finding constitutes a very good starting-point for further optimization of the studied process.
Synthesis and evaluation of new steroidal lactam conjugates with aniline mustards as potential antileukemic therapeutics
Trafalis, Dimitrios,Geromichalou, Elena,Dalezis, Panagiotis,Nikoleousakos, Nikolaos,Sarli, Vasiliki
, p. 1 - 8 (2016)
Alkylating agents are still nowadays one of the most important classes of cytotoxic drugs, which display a wide range of therapeutic use for the treatment of various cancers. We have synthesized and tested four hybrid homo-azasteroidal alkylating esters for antileukemic activity against five sensitive to alkylating agents human leukemia cell lines in vitro and against P388 murine leukemia in vivo. Comparatively, melphalan and 3-(4-(bis(2-chloroethyl)amino)phenoxy)propanoic acid (POPAM) were also examined. All the homo-aza-steroidal alkylators showed relatively lower acute toxicity, very promising and antileukemic activity both in vitro and in vivo.
Novel catalytic activity of immobilized spores under reduced water activity
Dutta, Tapan K.,Samanta, Timir B.
, p. 629 - 632 (1997)
Onset of a new catalytic function during transformation of progesterone by immobilized spores of Aspergillus ochraceus TS under reduced water activity is reported. The pathway of transformation, which furnished 1,4-androstadien-17β-ol-3-one and 1,4-androstadien-3,17-dione due to cleavage of C17-C20 bond, is different from normal reaction sequence.
Diastereoselective hydroformylation of Δ4-steroids with rhodium-phosphite catalysts
Freixa, Zoraida,Pereira, Mariette M.,Bayon,Silva, Artur M.S.,Salvador, Jorge A.R.,Beja, Ana Matos,Paixao, Jose A.,Ramos, Manuela
, p. 1083 - 1087 (2001)
The hydroformylation of two steroidal substrates, namely 17β-acetoxyandrost-4-ene 1 and 3β,17β-diacetoxyandrost-4-ene 2, with a rhodium tris(O-tert-butylphenyl)phosphite catalyst was investigated. In both cases, the major reaction product was 4β-formyl-17β-acetoxy-5β-androstane 3, which was isolated and characterized by X-ray diffraction and NMR techniques. This reaction is the first example of catalytic carbonylation to the β face of a steroid backbone. The effect of reaction temperature, the pressure at which the reaction was completed and the ligand:Rh ratio on the regio- and stereoselectivity of the reaction is also discussed.
Iridium-catalysed highly selective reduction-elimination of steroidal 4-en-3-ones to 3,5-dienes in water
Li, Jide,Tang, Weiping,Ren, Demin,Xu, Jiaxi,Yang, Zhanhui
supporting information, p. 2088 - 2094 (2019/04/29)
Steroidal 3,5-diene is an important structural motif in steroid drugs. In this report, an iridium-catalyzed reduction-elimination of readily available steroidal 4-en-3-ones is realized to prepare steroidal 3,5-dienes. At a low catalyst loading (S/C = 200), heating 4-en-3-ones in a water-mixed organic solvent with formic acid without inert atmosphere protection afforded the desired 3,5-dienes in moderate to excellent yields. In a gram-scale preparation, recrystallization is used instead of column chromatography to purify products. Excellent functionality tolerance and regioselectivity are featured. Structural moieties such as alkanols (primary, secondary and tertiary), esters (except for formate), tolylates, and ketones (endocyclic or exocyclic) are not affected. Surprisingly, the reduction-elimination only takes place at A-ring 4-en-3-ones. In addition, bicyclic 4-en-3-ones are also viable substrates. Synthetic applications of steroidal 3,5-dienes are demonstrated. Our method can also lead to steroidal 3,5-dienes-3-d (>99% d-incorporation) when DCO2D and D2O are used together.
Selective reduction of 4,6- conjugate diene -3-one steroid compound method
-
Paragraph 0066-0073, (2019/11/21)
Belonging to the field of chemical pharmacy, the invention relates to a method for selective reduction of 4, 6-conjugated diene-3-one steroid, and solves the problem of low yield in hydrogen reduction. The method mainly includes the steps of: 1) adding the 4, 6-conjugated diene-3-one steroid, a liquid solvent, a catalyst, and a reducing agent hydrogen donor into a reaction kettle, performing nitrogen protection, and carrying out stirring heating till reflux; 2) carry out reflux reaction for 3-10h; 3) at the end of reaction, filtering out the catalyst; 4) distilling the solvent; 5) adding purified water after distillation; and 6) conducting cooling, pumping filtering, washing and drying to obtain a 4-ene-3-steroid crystal.
1045-69-8 Process route
-
-
57-83-0
Progesterone

-
-
pregnan-3-ol-20-one

-
-
4479-11-2
pregnane-3,20-diol

-
-
15114-79-1
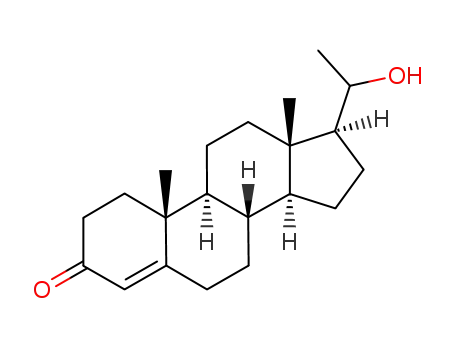
4-pregnen-20α-ol-3-one

-
-
58-22-0
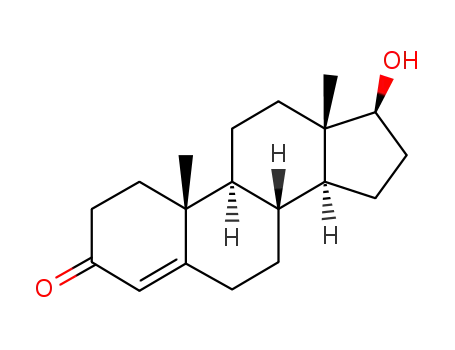
testosterone

-
-
566-65-4
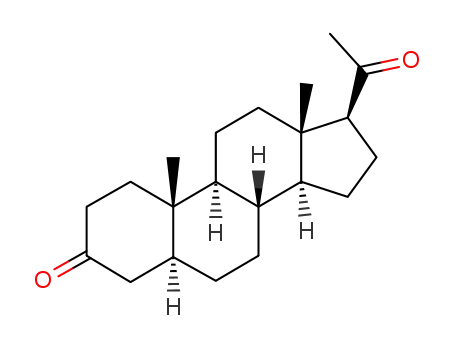
dihydroprogesterone

-
-
63-05-8
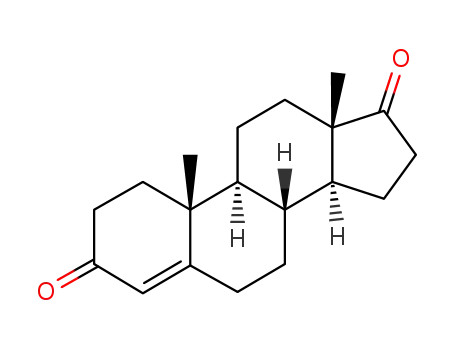
Androstenedione

-
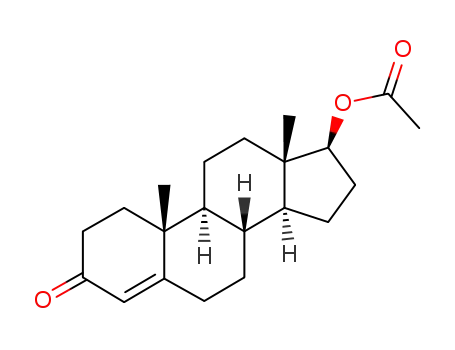
-
1045-69-8
testosterone acetate
| Conditions | Yield |
|---|---|
|
With
Hortaea werneckii B-763 at late logarithmic and stationary growth phase;
In
YNB growth medium; N,N-dimethyl-formamide;
at 28 ℃;
for 24h;
Enzymatic reaction;
|
-
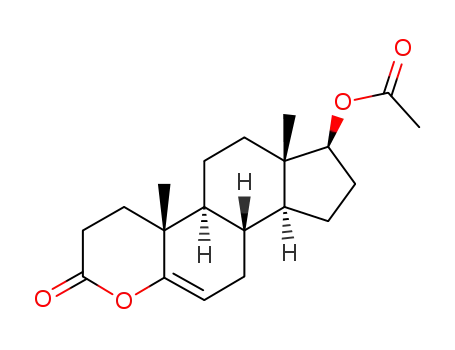
-
1458-92-0
17β-acetoxy-4-oxa-androst-5-en-3-one

-
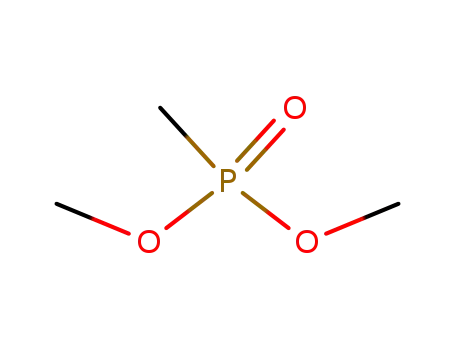
-
756-79-6
dimethyl methane phosphonate

-

-
58-22-0
testosterone

-

-
1045-69-8
testosterone acetate
| Conditions | Yield |
|---|---|
|
Yield given. Multistep reaction. Yields of byproduct given;
|
1045-69-8 Upstream products
-
52091-98-2
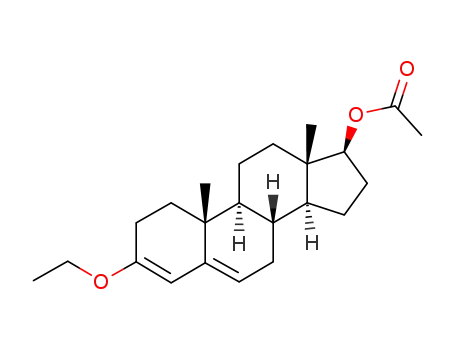
17β-acetoxy-3-ethoxy-androsta-3,5-diene
-
111294-86-1
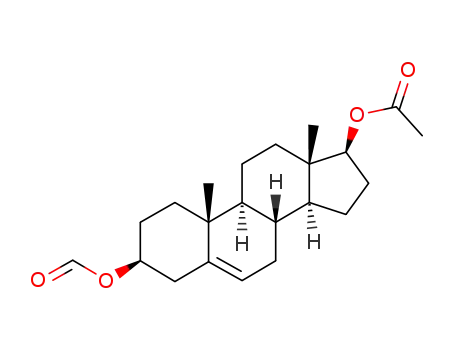
17β-acetoxy-3β-formyloxy-androst-5-ene
-
97922-54-8
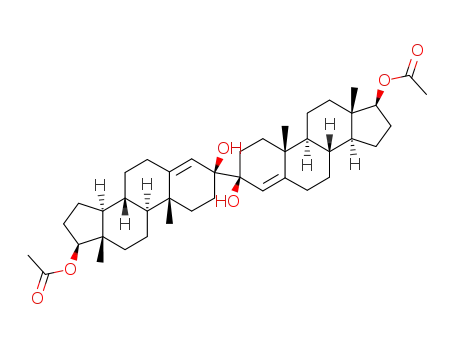
3β.3'β-dihydroxy-17β.17β'-diacetoxy-[3.3']bi[androsten-(4)-yl]
-
911651-92-8
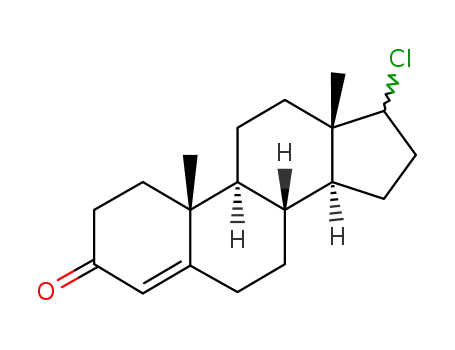
17-chloroandrost-4-en-3-one
1045-69-8 Downstream products
-
1778-93-4
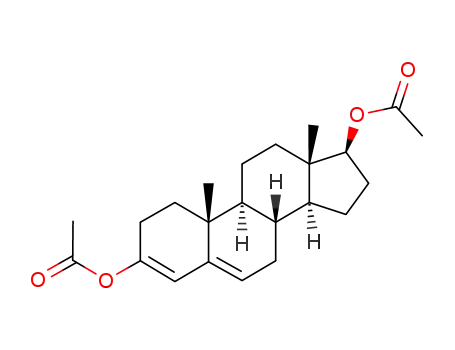
testosterone enol diacetate
-
58-22-0

testosterone
-
2352-19-4
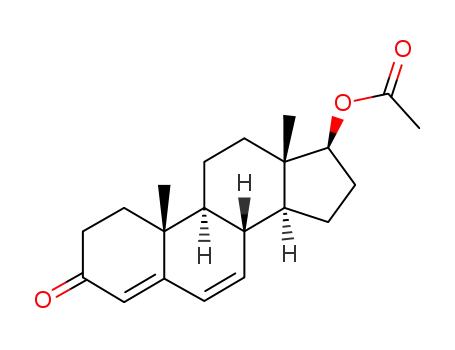
androsta-4,6-dien-17β-ol-3-one acetate
-
1759-35-9
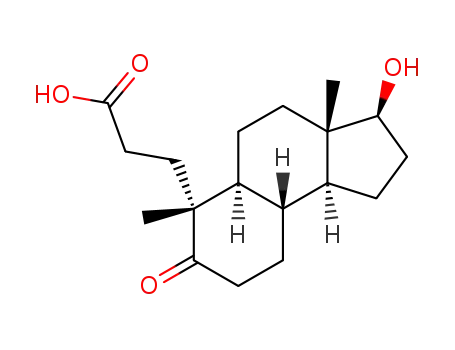
5-oxo-A-nor-3,5-seco-17β-hydroxy-androstan-3-oic acid








