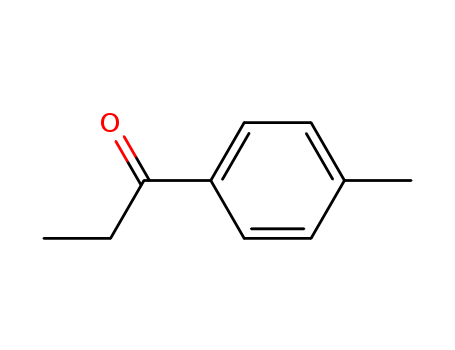
4-Methylpropiophenone 5337-93-9
- CasNo:5337-93-9
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
5337-93-9 Properties
- Molecular Formula:C10H12O
- Molecular Weight:148.205
- Appearance/Colour:clear light yellow liquid
- Vapor Pressure:0.0423mmHg at 25°C
- Melting Point:7.2 °C
- Refractive Index:n20/D 1.528(lit.)
- Boiling Point:238.5 °C at 760 mmHg
- Flash Point:97.9 °C
- PSA:17.07000
- Density:0.963 g/cm3
- LogP:2.58770
5337-93-9 Usage
Chemical Properties
clear light yellow liquid
Uses
4'-methylpropiophenone has been reported to be used in the synthesis of pharmaceutical compounds such as tolperisone and other muscle relaxants. The substance is also a starting material in the synthesis of 4-methylmethcathinone (mephedrone). Mephedrone has been encountered as a ‘legal high’ and on the illicit drug market masquerading as cocaine (in powder form), MDMA (in tablet form) and as an adulterant. According to the 2016 European Drugs Market Report produced by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), mephedrone has created a specific demand and carved its own distinct market share.
Uses
4′-Methylpropiophenone is a chemical reagent used in electrocarboxylation reactions.
Application
4′-Methylpropiophenone can be used as a building block to synthesize:N-(4-Chlorophenyl)-N′-[1-(4-methylphenyl)propyl]urea as a potential CRAC channel inhibitor.Mono and trinuclear cobaloxime/organocobaloxime complexes, which are used as efficient catalysts for the synthesis of cyclic carbonates.Tolperisone (TOL) via acid-catalyzed reaction with piperidine hydrochloride and 1,2-dioxolane.
Definition
ChEBI: 4-methylpropiophenone is an aromatic ketone that is propiophenone bearing a methyl group at C-4. It derives from a propiophenone.
InChI:InChI=1/C10H12O/c1-3-10(11)9-6-4-8(2)5-7-9/h4-7H,3H2,1-2H3
5337-93-9 Relevant articles
α-Hydroxylation of α,α-Disubstituted N-tert-Butanesulfinyl Ketimines with Molecular Oxygen: Stereoselective Synthesis of α-Tertiary Hydroxyimines
Liu, Hui,Lu, Chong-Dao,Yao, Yun,Yisimayili, Nuermaimaiti
supporting information, (2022/01/20)
α-Tertiary hydroxyimines were stereoselectively synthesized from enantioenriched N-tert-butanesulfinyl ketimines using potassium tert-butoxide, molecular oxygen, and trimethyl phosphite. The stereoselective hydroxylation of acyclic ketimines bearing two sterically similar α-substituents was achieved by controlling the geometry of the metalloenamine intermediates and the facial selectivity of hydroxylation. The synthetic utility of the resulting α-tertiary hydroxyimines was demonstrated through the successful diastereoselective synthesis of highly substituted β-amino alcohols.
Photoredox/nickel-catalyzed hydroacylation of ethylene with aromatic acids
Chen, Shuai,He, Hengchi,Li, Weipeng,Xie, Jin,Zhang, Lili,Zhu, Chengjian
supporting information, p. 9064 - 9067 (2021/09/15)
We report a general, practical and scalable hydroacylation reaction of ethylene with aromatic carboxylic acids with the synergistic combination of nickel and photoredox catalysis. Under ambient temperature and pressure, feedstock chemicals such as ethylene can be converted into high-value-added aromatic ketones in moderate to good yields (up to 92%) with reaction time of 2-6 hours.
Cobalt-Catalyzed Reductive C-O Bond Cleavage of Lignin β-O-4 Ketone Models via in Situ Generation of the Cobalt-Boryl Species
Gao, Kecheng,Xu, Man,Cai, Cheng,Ding, Yanghao,Chen, Jianhui,Liu, Bosheng,Xia, Yuanzhi
, p. 6055 - 6060 (2020/08/12)
An efficient and mild method for reductive C-O bond cleavage of lignin β-O-4 ketone models was developed to afford the corresponding ketones and phenols with PDI-CoCl2 as the precatalyst and diboron reagent as the reductant. The synthetic utility of the methodology was demonstrated by depolymerization of a polymeric model and gram-scale transformation. Mechanistic studies suggested that this transformation involves steps of carbonyl insertion, 1,2-Brook type rearrangement, β-oxygen elimination, and rate-limiting regeneration of the catalytic active Co-B species.
Metal-Organic Framework Based on Heptanuclear Cu-O Clusters and Its Application as a Recyclable Photocatalyst for Stepwise Selective Catalysis
Zhou, Jie,Huang-Fu, Xu,Huang, Yang-Ying,Cao, Chu-Ning,Han, Jie,Zhao, Xiao-Li,Chen, Xu-Dong
, p. 254 - 263 (2019/12/04)
Visible-light driven photoreactions using metal-organic frameworks (MOFs) as catalysts are promising with regard to their environmental friendly features such as the use of renewable and sustainable energy of visible light and potential catalyst recyclability. To develop potential heterogeneous photocatalysts, a family of three copper(II) coordination polymers bearing different Cu-O assemblies have been synthesized with the ligand 4,4-disulfo-[1,1-biphenyl]-2,2-dicarboxylate acid (H4DSDC), namely, {[Cu7(DSDC)2(OH)6(H2O)10]·xH2O}n (1), {[Cu4(DSDC)(4,4-bpy)2(OH)4]·2H2O}n (2), and {Cu2(DSDC)(phen)2(H2O)2}n (3) (4,4-bpy = 4,4-bipyridine and phen = 1,10-phenanthroline). Complex 1 represents a metal-organic framework featuring a NbO type topology constructed from the infinite linkage of heptanuclear [Cu7(μ3-OH)6(H2O)10]8+ clusters by deprotonated DSDC4- ligands, comprising one-dimensional hexagonal channels of a diameter around 11 ? that are filled with water molecules. The infinite waving {[Cu2(OH)2]2+}n ladderlike chains in complex 2 are bridged by DSDC4- and 4,4-bpy ligands into a three-dimensional framework. A two-dimensional layered structure is formed in complex 3 due to the existence of terminal phenanthroline ligands. All of the coordination polymers 1-3 are able to catalyze the visible-light driven oxidation of alcohols at mild conditions using hydrogen peroxide as an oxidant, in which complex 1 demonstrates satisfactory efficiency. Significantly for this photoreaction catalyzed by 1, the extent of oxidation over aryl primary alcohols is fully controllable with time-resolved product selectivity, giving either corresponding aldehydes or carboxylate acids in good yields. It is also remarkable that the photocatalyst could be recovered almost quantitatively on completion of the catalytic cycle without any structure change, and could be recycled for catalytic use for at least five cycles with constant efficiency. This photocatalyst with time-resolved selectivity for different products may provide new insight into the design and development of novel catalytic systems.
5337-93-9 Process route
-
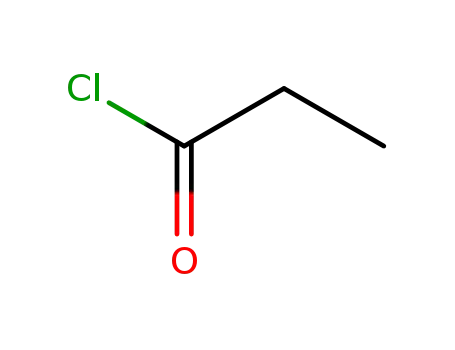
-
79-03-8
propionyl chloride

-
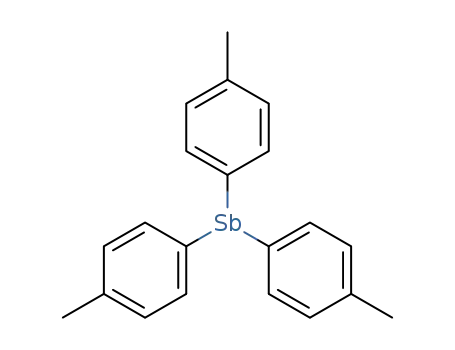
-
5395-43-7
tri(p-tolyl)antimony

-
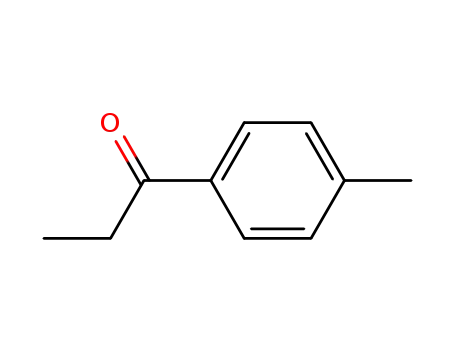
-
5337-93-9
4'-methylpropiophenone

-
-
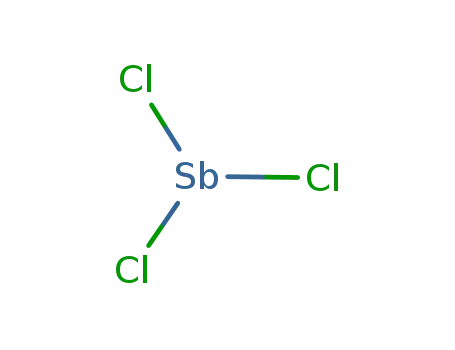
10025-91-9
antimony(III) chloride
| Conditions | Yield |
|---|---|
|
With
aluminium trichloride;
In
carbon disulfide;
byproducts: Al(OH)3; hydrolysis;
|
60-75 |
|
|
-
-
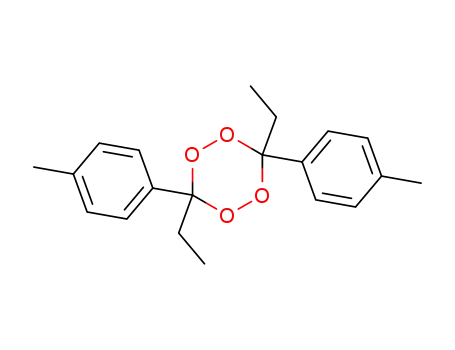
74783-32-7
trans-3,6-diethyl-3,6-di-p-tolyl-1,2,4,5-tetraoxan

-
-
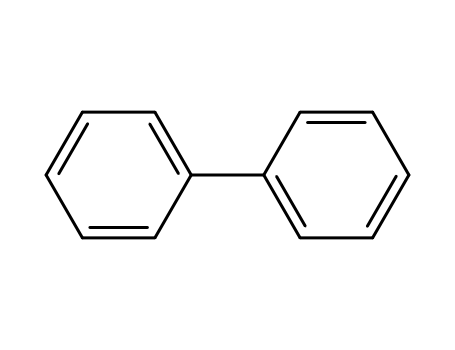
92-52-4,1594-86-1
biphenyl

-
-
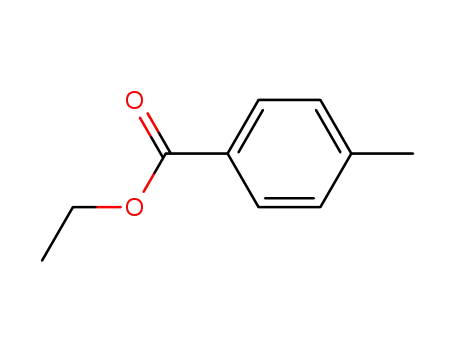
94-08-6
4-methylbenzoic acid ethyl ester

-
-
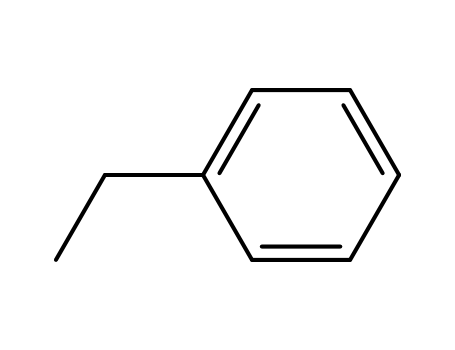
100-41-4,27536-89-6
ethylbenzene

-
-
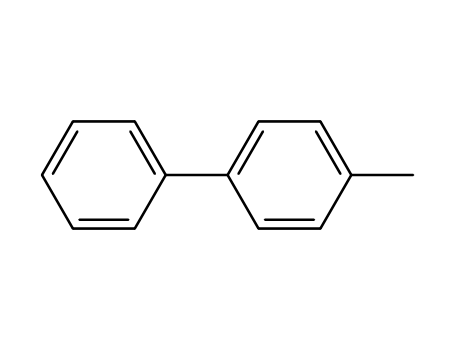
644-08-6
4-Methylbiphenyl

-

-
5337-93-9
4'-methylpropiophenone
| Conditions | Yield |
|---|---|
|
In
benzene;
at 155 ℃;
for 87h;
Product distribution;
Mechanism;
thermolysis;
|
4% |
5337-93-9 Upstream products
-
2040-14-4
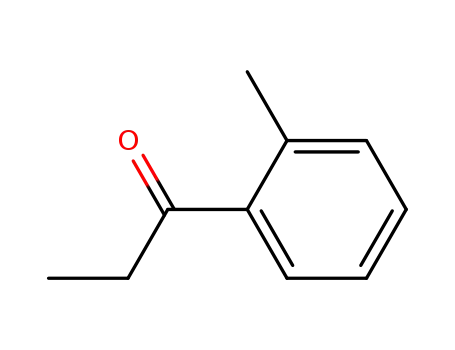
2-methylpropiophenone
-
25574-04-3
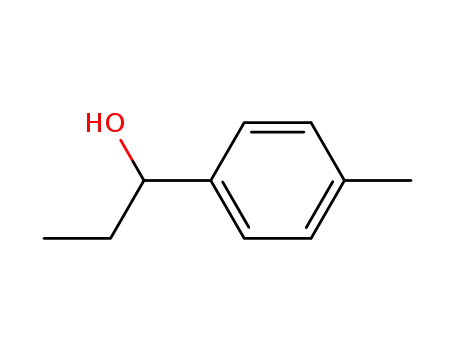
1-p-tolyl-1-propanol
-
79-03-8

propionyl chloride
-
108-88-3
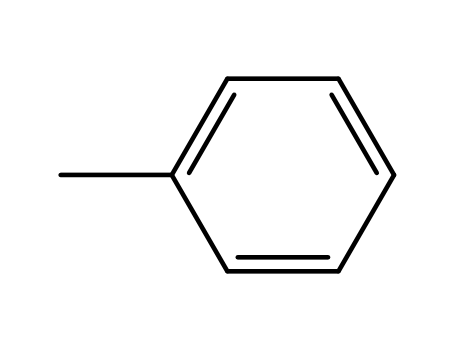
toluene
5337-93-9 Downstream products
-
92207-33-5
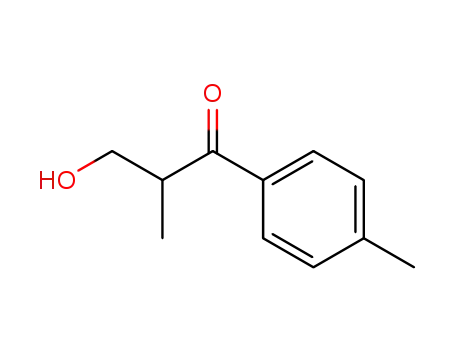
3-hydroxy-2-methyl-1-(p-tolyl)propan-1-one
-
5400-87-3
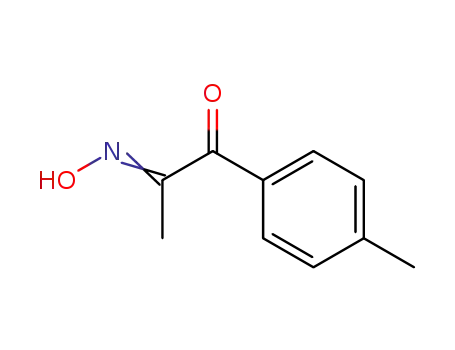
1-p-tolyl-propane-1,2-dione-2-oxime
-
937371-82-9
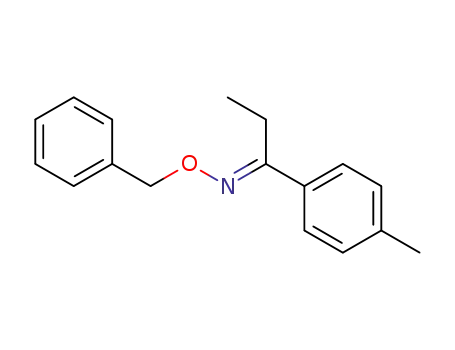
(E)-1-(4-methylphenyl)propanone O-benzyl oxime
-
62834-90-6
2,4-dimethyl-1-p-tolyl-pent-4-en-1-one








