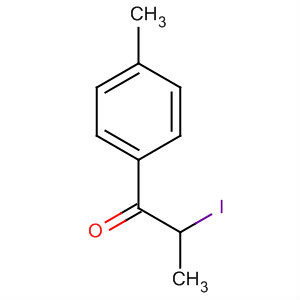
2-iodo-1-p-tolylpropan-1-one 236117-38-7
- CasNo:236117-38-7
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
236117-38-7 Properties
- Molecular Formula:C10H11IO
- Molecular Weight:274.09800
- PSA:17.07000
- LogP:3.00120
236117-38-7 Relevant articles
α,α-Alkylation-Halogenation and Dihalogenation of Sulfoxonium Ylides. A Direct Preparation of Geminal Difunctionalized Ketones
Gallo, Rafael D. C.,Ahmad, Anees,Metzker, Gustavo,Burtoloso, Antonio C. B.
, p. 16980 - 16984 (2017/11/27)
A one-pot alkylation–halogenation of ketosulfoxonium ylides in the presence of alkyl halides is described. The method furnishes several gem-difunctionalized haloketones (an alkyl and F, Cl, Br, or I) in good yields. Replacing alkyl halides with a mixture of electrophilic halogen species and various halide anions led to gem-dihalogenated ketones containing a combination of the same or two different halogens. Kinetic isotopic effects as well as reaction kinetic experiments give insight to the mechanism of these reactions.
Direct metal-free α-iodination of arylketones induced by iodine or iodomethane with HTIB in ionic liquid
Lee, Jong Chan,Kim, Jimi,Park, Hyun Jung,Kwag, Byungmook,Lee, Seung Bae
experimental part, p. 1385 - 1386 (2010/09/18)
-
Efficient α-iodination of carbonyl compounds under solvent-free conditions using microwave irradiation
Lee, Jong Chan,Bae, Yong Hun
, p. 507 - 508 (2007/10/03)
Direct conversion of carbonyl compounds into α-iodocarbonyl compounds has been successfully achieved under solvent-free microwave irradiation conditions using N-iodosuccinimide and p-toluenesulfonic acid.
Efficient method for α-iodination of ketones
Lee, Jong Chan,Jin, Yong Suk
, p. 2769 - 2774 (2007/10/03)
α-Iodoketones are prepared in high yields from the initial reaction of various ketones with HNIB in CH3CN and subsequent treatment of potassium iodide or samarium(II) iodide.
236117-38-7 Process route
-
-
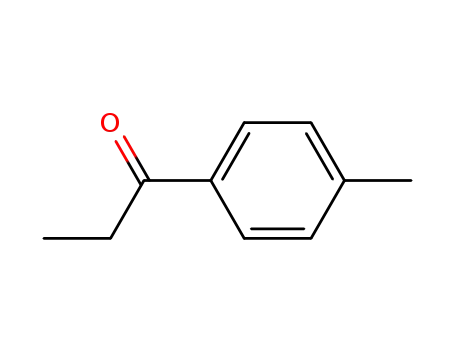
5337-93-9
4'-methylpropiophenone

-
-
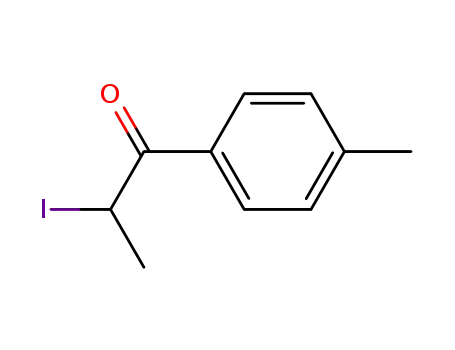
236117-38-7
2-iodo-1-(p-tolyl)propan-1-one
| Conditions | Yield |
|---|---|
|
With
N-iodo-succinimide; toluene-4-sulfonic acid;
Microwave irradiation;
|
90% |
|
With
[hydroxy(tosyloxy)iodo]benzene; iodine; 1-butyl-3-methylimidazolium Tetrafluoroborate;
at 60 ℃;
Ionic liquid;
|
84% |
-
-
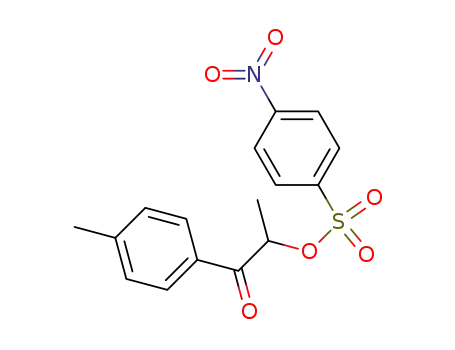
4-Nitro-benzenesulfonic acid 1-methyl-2-oxo-2-p-tolyl-ethyl ester

-

-
236117-38-7
2-iodo-1-(p-tolyl)propan-1-one
| Conditions | Yield |
|---|---|
|
With
18-crown-6 ether; potassium iodide;
In
acetonitrile;
at 20 ℃;
for 1h;
|
236117-38-7 Upstream products
-
92821-88-0
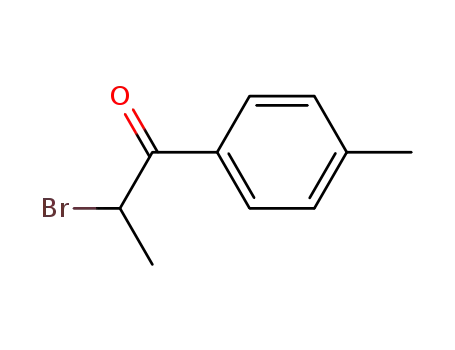
2-bromo-4'-methylpropiophenone
-
5337-93-9

4'-methylpropiophenone
-
74-88-4
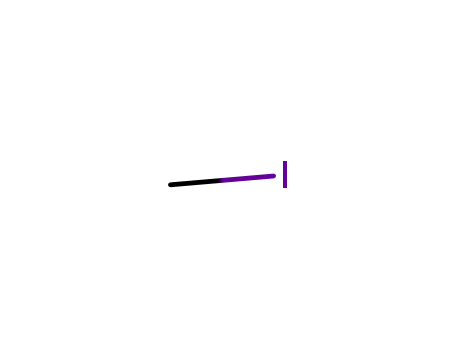
methyl iodide
-
874-60-2
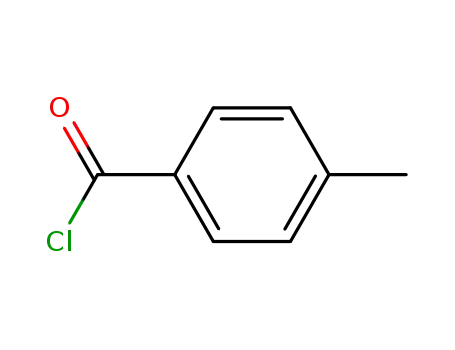
4-methyl-benzoyl chloride








