Product Details
159752-10-0 Properties
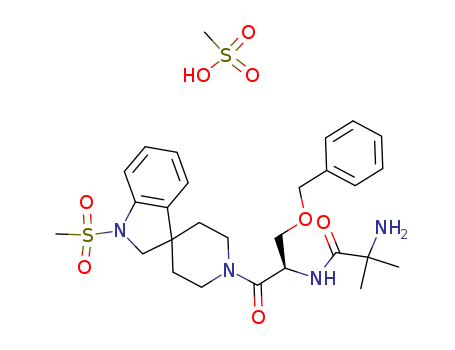
- Molecular Formula:CH4O3S*C27H36N4O5S
- Molecular Weight:624.78
- Boiling Point:868.9 °C at 760 mmHg
- Flash Point:479.3 °C
- PSA:196.66000
- LogP:4.97440
159752-10-0 Usage
Description
MK-677?(also known as ibutamoren),?promotes the secretion of the growth hormone (GH)?and?increases insulin-like growth factor 1 (IGF-1). Ibutamoren increases growth hormone levels by mimicking the action of the hormone?ghrelin?and binding to one of the ghrelin receptors (GHSR) in the brain. Activated GHSR stimulates growth hormone release from the brain. Clinical studies describe only the effects ibutamoren has on?appetite and as expected, like ghrelin, ibutamoren increases it. GHSR is found in brain regions that control appetite, pleasure, mood, biological rhythms, memory, and cognition.
Benefits
The benefits of MK-677 include muscle-building, a reduction in muscle wasting, better bone density, improved sleep, and anti-aging properties. It may also have nootropic effects and it can be beneficial in treating growth hormone deficiencies. Read on for a more in-depth look at these benefits.
Uses
Growth hormone releasing factor.MK-677 is a potent, non-peptide ghrelin receptor agonist.
Biochem/physiol Actions
It is also known as ibutamoren mesylate. MK-677 is a mimic of growth hormone (GH)-releasing peptide and is orally active.
159752-10-0 Relevant articles
Domino Aryne Annulation via a Nucleophilic-Ene Process
Xu, Hai,He, Jia,Shi, Jiarong,Tan, Liang,Qiu, Dachuan,Luo, Xiaohua,Li, Yang
, p. 3555 - 3559 (2018/03/21)
1,2-Benzdiyne equivalents possess the unique property that they can react with two arynophiles through iteratively generated 1,2- and 2,3-aryne intermediates. Upon rational modification on the second leaving group of these aryne precursors, a domino aryne annulation approach was developed through a nucleophilic-ene reaction sequence. Various benzo-fused N-heterocyclic frameworks were achievable under transition metal-free conditions with a broad substrate scope.
A facile synthesis of the spiroindoline-based growth hormone secretagogue, MK-677
Qi, Xian Liang,Yang, Er Qun,Zhang, Jun Tao,Wang, Tao,Cao, Xiao Ping
, p. 661 - 664 (2012/07/03)
A facile and improved route for the synthesis of the orally active spiroindoline-based growth hormone secretagogue, MK-677 was described. The key step adopted the Fischer indole/reduction strategy. The preparation of the key intermediates N-protected piperidine carboxaldehyde 5 and the N-Boc-O-benzyl-d-serine (2) are also optimized.
Polymorphic forms of a growth hormone secretagogue
-
, (2008/06/13)
This invention is concerned with polymorphic forms of the compound N-?1(R)-?(1,2-dihydro-1-methanesulfonylspiro?3H-indole-3,4'-piperdin!-1'-yl)carbonyl!-2-(phenylmethyloxy)ethyl!-2-amino-2-methylpropanamide methanesulfonate which is a growth hormone secretagogue that is useful in food animals to promote their growth thereby rendering the production of edible meat products more efficient, and in humans, to treat physiological or medical conditions characterized by a deficiency in growth hormone secretion, and to treat medical conditions which are improved by the anabolic effects of growth hormone. The instant polymorphic forms have advantages over the other known forms of N-?1(R)-?(1,2-dihydro-1-methanesulfonylspiro?3H-indole-3,4'-piperdin!-1'-yl)carbonyl!-2-(phenylmethyloxy)-ethyl!-2-amino-2-methylpropanamide methanesulfonate in terms of thermodynamic stability and suitability for inclusion in pharmaceutical formulations. The present invention is also concerned with processes for preparing these polymorphic forms, pharmaceutical formulations comprising these polymorphic forms as active ingredients and the use of the polymorphic form of the compound and their formulations in the treatment of certain disorders.
Synthesis of the orally active spiroindoline-based Growth Hormone Secretagogue, MK-677
Maligres, Peter E.,Houpis, Ioannis,Rossen, Kai,Molina, Audrey,Sager, Jess,Upadhyay, Veena,Wells, Kenneth M.,Reamer, Robert A.,Lynch, Joseph E.,Askin, David,Volante,Reider, Paul J.,Houghton, Peter
, p. 10983 - 10992 (2007/10/03)
The preparation of the Merck Growth Hormone Secretagogue; MK-677 is described. A Fischer indole/reduction based strategy provides the novel spiroindoline nucleus of this potent compound. This optimized sequence necessitates the isolation of only one intermediate 10 and provides MK-677 in 48% overall yield from isonipecotic acid 3.
159752-10-0 Process route
-
-
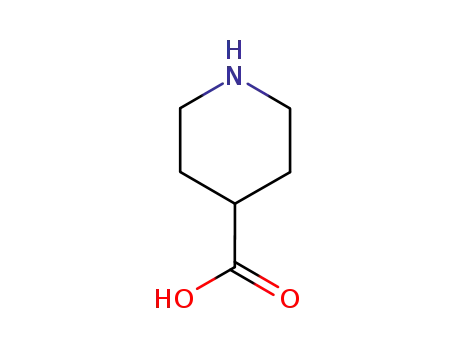
498-94-2
isonipecotic acid

-
-
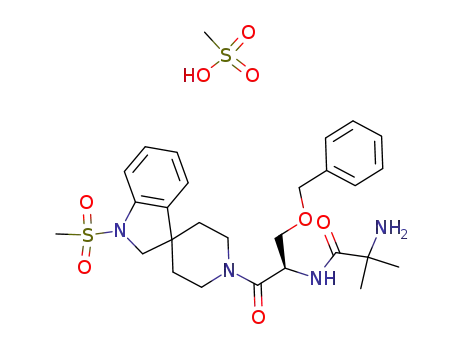
191487-52-2,214962-40-0,159752-10-0
MK-677
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 12 steps
1: 97 percent / K2CO3 / H2O / 58 h / 22 °C
2: (COCl)2 / toluene; dimethylformamide / 16 h / 18 °C
3: 94 percent / H2, DIEA, thioanisole / Pd/C / toluene / 22 h / 20 °C / 2068.6 Torr
4: 99 percent / TFA / CH2Cl2 / 17 h / 35 °C
5: NaBH4 / toluene / 0.5 h / -2 °C
6: DIEA / tetrahydrofuran / 2 h / 5 - 8 °C
7: 93 percent / H2 / Pd-C / ethanol / 5 h / 65 °C / 2068.6 Torr
8: DCC, HOBt / H2O; various solvent(s) / 5 h / Ambient temperature
9: MsOH / ethanol / 7.5 h / 35 - 40 °C
10: DCC, HOBt / various solvent(s) / 2 h / Ambient temperature
11: MsOH / ethanol / 35 - 40 °C
12: ethanol; ethyl acetate / 55 °C
With
sodium tetrahydroborate; oxalyl dichloride; methanesulfonic acid; methyl-phenyl-thioether; hydrogen; potassium carbonate; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide; trifluoroacetic acid;
palladium on activated charcoal;
In
tetrahydrofuran; ethanol; dichloromethane; water; ethyl acetate; N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 10 steps
1: lithium aluminium tetrahydride / tetrahydrofuran / 20 °C
2: potassium carbonate / 20 °C
3: pyridinium chlorochromate / dichloromethane / 20 °C
4: trifluoroacetic acid / dichloromethane
5: sodium tetrahydroborate / methanol
6: N-ethyl-N,N-diisopropylamine / tetrahydrofuran
7: palladium 10% on activated carbon; hydrogen / ethanol
8: benzotriazol-1-ol; dicyclohexyl-carbodiimide / Isopropyl acetate; water
9: benzotriazol-1-ol; dicyclohexyl-carbodiimide / Isopropyl acetate; water
10: ethanol
With
sodium tetrahydroborate; lithium aluminium tetrahydride; palladium 10% on activated carbon; hydrogen; potassium carbonate; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide; pyridinium chlorochromate; trifluoroacetic acid;
In
tetrahydrofuran; methanol; ethanol; dichloromethane; Isopropyl acetate; water;
|
-
-
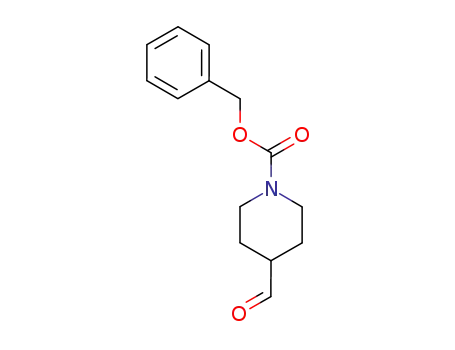
138163-08-3
N-(benzyloxycarbonyl)piperidine-4-carboxaldehyde

-

-
191487-52-2,214962-40-0,159752-10-0
MK-677
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
1: 99 percent / TFA / CH2Cl2 / 17 h / 35 °C
2: NaBH4 / toluene / 0.5 h / -2 °C
3: DIEA / tetrahydrofuran / 2 h / 5 - 8 °C
4: 93 percent / H2 / Pd-C / ethanol / 5 h / 65 °C / 2068.6 Torr
5: DCC, HOBt / H2O; various solvent(s) / 5 h / Ambient temperature
6: MsOH / ethanol / 7.5 h / 35 - 40 °C
7: DCC, HOBt / various solvent(s) / 2 h / Ambient temperature
8: MsOH / ethanol / 35 - 40 °C
9: ethanol; ethyl acetate / 55 °C
With
sodium tetrahydroborate; methanesulfonic acid; hydrogen; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide; trifluoroacetic acid;
palladium on activated charcoal;
In
tetrahydrofuran; ethanol; dichloromethane; water; ethyl acetate; toluene;
|
|
|
Multi-step reaction with 7 steps
1: trifluoroacetic acid / dichloromethane
2: sodium tetrahydroborate / methanol
3: N-ethyl-N,N-diisopropylamine / tetrahydrofuran
4: palladium 10% on activated carbon; hydrogen / ethanol
5: benzotriazol-1-ol; dicyclohexyl-carbodiimide / Isopropyl acetate; water
6: benzotriazol-1-ol; dicyclohexyl-carbodiimide / Isopropyl acetate; water
7: ethanol
With
sodium tetrahydroborate; palladium 10% on activated carbon; hydrogen; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide; trifluoroacetic acid;
In
tetrahydrofuran; methanol; ethanol; dichloromethane; Isopropyl acetate; water;
|
159752-10-0 Upstream products
-
498-94-2

isonipecotic acid
-
47173-80-8
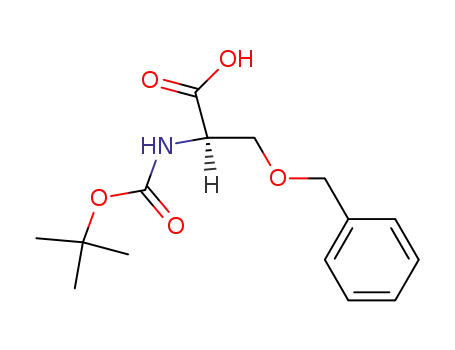
N-Boc-D-serine(Bzl)-OH
-
100-39-0
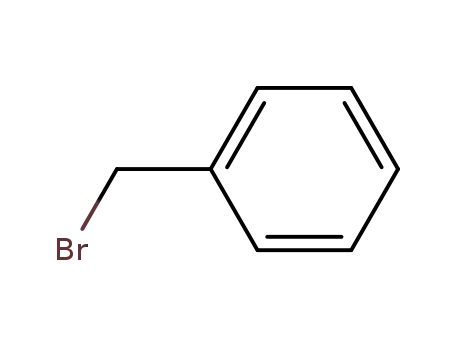
benzyl bromide
-
10314-98-4
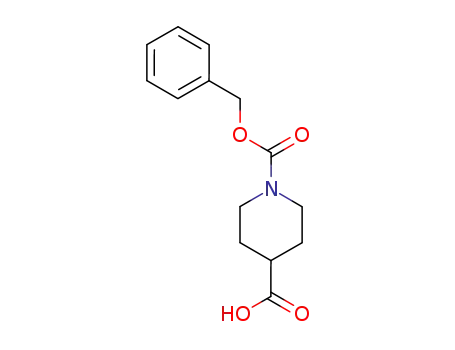
1-benzyloxycarbonylpiperidine-4-carboxylic acid








