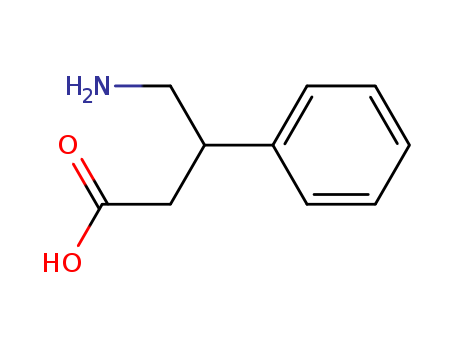
4-Amino-3-phenylbutyric acid hydrochloride 1078-21-3
- CasNo:1078-21-3
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
1078-21-3 Properties
- Molecular Formula:C10H13NO2
- Molecular Weight:179.219
- Appearance/Colour:white to off-white crystalline powder
- Vapor Pressure:7.98E-05mmHg at 25°C
- Melting Point:194.0-202 °C
- Refractive Index:1.5710 (estimate)
- Boiling Point:327.8 °C at 760 mmHg
- PKA:4.10±0.10(Predicted)
- Flash Point:152.1 °C
- PSA:63.32000
- Density:1.161 g/cm3
- LogP:2.70590
1078-21-3 Usage
Description
Phenibut, also known as fenibut, phenigamma, and beta-phenyl-gamma-aminobutyric acid, is a derivative of the neurotransmitter GABA. It was developed in Russia,and there it has been used clinically since the 1960s to treat anxiety and related conditions,including insomnia(Lapin,2001). Phenibut has anxiolytic properties and is commonlycompared to benzodiazepines and baclofen.Structurally,phenibut issimilar to GABA, with the addition of the phenyl ring. This allows thecompound to more easily cross the blood-brain barrier, but also changesits activity profile (Shulgina,1986). Scientific studies demonstrate that phenibut can be safely used to treat anxiety, depression,epilepsy, speech disorders, and insomnia.
Uses
4-amino-3-phenylbutanoic acid (cas# 1078-21-3) is a GABAA receptor agonist, used for treatment of disorders influenced by dysfunction of pancreatic β cells and also used in combination with other agents.
Preparation
Phenibut was synthesized by Perekalin and his associates at the Department of Organic Chemistry of the Herzen Pedagogic Institute in St. Petersburg, the Russian Federation. In initial publications phenibut was known as phenigamma (Lapin, 2001, V. V. Perekalin, 1954).Fenibut synthesis: from benzoyl chloride and ethyl acetoacetate as raw materials, through condensation and hydrolysis of ethyl benzoyl acetate, and then condensation of benzaldehyde and nitromethane to generate trans-nitrostyrene, and then the two are combined. Micheal addition, the adduct is then catalytically hydrogenated with Raney nickel, and finally hydrolyzed in concentrated hydrochloric acid to produce phenibate.Synthesis of Phenibut (16) and Baclofen (17).
Application
Phenibut is an anxiolytic and nootropic drug, discovered in the Soviet Union used to treat several psychiatric disorders. It can be used in the treatment of anxiety, depression, asthenia, post-traumatic stress disorder, stuttering, and vestibular disorders.
Safety Profile
Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: somnolence, hallucinations, distorted perception. Used as a mood elevator and tranquilizer. When heated to decomposition it emits toxic fumes of NOx.
Regulatory Status
USA - Phenibut is still an uncontrolled substance in the United States, and it is legal to sell and possess phenibut. It has not been approved for clinical use in the United States. Europe - Unscheduled. It is not regulated by the European Medicines Agency. However, some nootropic substances such as piracetam are available only on prescription in some European Union countries and are freely available in others. Russia - Phenibut is a licensed prescription medication used for a variety of conditions including anxiety, insomnia, post-traumatic stress disorders, depression, stuttering, tics, attention deficit disorders, and vestibular disorders. Russian cosmonauts were reported to have been supplied with the substance to help relieve tension, anxiety and fear (Buckley 2006).
InChI:InChI=1/C10H13NO2.ClH/c11-7-9(6-10(12)13)8-4-2-1-3-5-8;/h1-5,9H,6-7,11H2,(H,12,13);1H
1078-21-3 Relevant articles
Highly Stable Zr(IV)-Based Metal-Organic Frameworks for Chiral Separation in Reversed-Phase Liquid Chromatography
Jiang, Hong,Yang, Kuiwei,Zhao, Xiangxiang,Zhang, Wenqiang,Liu, Yan,Jiang, Jianwen,Cui, Yong
, p. 390 - 398 (2021)
Separation of racemic mixtures is of great importance and interest in chemistry and pharmacology. Porous materials including metal-organic frameworks (MOFs) have been widely explored as chiral stationary phases (CSPs) in chiral resolution. However, it remains a challenge to develop new CSPs for reversed-phase high-performance liquid chromatography (RP-HPLC), which is the most popular chromatographic mode and accounts for over 90% of all separations. Here we demonstrated for the first time that highly stable Zr-based MOFs can be efficient CSPs for RP-HPLC. By elaborately designing and synthesizing three tetracarboxylate ligands of enantiopure 1,1′-biphenyl-20-crown-6, we prepared three chiral porous Zr(IV)-MOFs with the framework formula [Zr6O4(OH)8(H2O)4(L)2]. They share the same flu topological structure but channels of different sizes and display excellent tolerance to water, acid, and base. Chiral crown ether moieties are periodically aligned within the framework channels, allowing for stereoselective recognition of guest molecules via supramolecular interactions. Under acidic aqueous eluent conditions, the Zr-MOF-packed HPLC columns provide high resolution, selectivity, and durability for the separation of a variety of model racemates, including unprotected and protected amino acids and N-containing drugs, which are comparable to or even superior to several commercial chiral columns for HPLC separation. DFT calculations suggest that the Zr-MOF provides a confined microenvironment for chiral crown ethers that dictates the separation selectivity.
Rhodium-catalyzed asymmetric hydrogenation of β-cyanocinnamic esters with the assistance of a single hydrogen bond in a precise position
Li, Xiuxiu,You, Cai,Yang, Yusheng,Yang, Yuhong,Li, Pan,Gu, Guoxian,Chung, Lung Wa,Lv, Hui,Zhang, Xumu
, p. 1919 - 1924 (2018)
With the assistance of hydrogen bonds, the first asymmetric hydrogenation of β-cyanocinnamic esters is developed, affording chiral β-cyano esters with excellent enantioselectivities (up to 99% ee). This novel methodology provides an efficient and concise synthetic route to chiral GABA-derivatives such as (S)-Pregabalin, (R)-Phenibut, (R)-Baclofen. Interestingly, in this system, the catalyst with a single H-bond donor performs better than that with double H-bond donors, which is a novel discovery in the metalorganocatalysis area.
Synthesis and Biological Analysis of Anti-addiction Effect and Hepatotoxicity of Tow Baclofen Analogues Complexed with β-Cyclodextrin
Dib, Mohammed El Amine,El Ouar, Ibtissem,Keniche, Assia,Zeghina, Ibtissem
, p. 187 - 196 (2022/02/02)
Aim and Objective: The excessive consumption of alcohol and the installation of dependence is, in most cases, facilitated by favorable psychological factors that trigger and maintain the behavior of consumers. Examples more frequently encountered in individuals having difficulty with alcohol are, in particular: one or more anxiety disorders, deficits in the capacities to manage stress and anxiety. The main objective of this work was to study in vivo the anti-addiction effect and hepatotoxicity of tow baclofen analogues complexed with β-Cyclodextrin (βCD) on an alcohol-dependent rat model. Materials and Methods: The synthesis of two analogues, ABF1 and ABF2, close to baclofen was reported. The structural determination of the two compounds was confirmed by NMR and IR analysis. The complexation of analogues with β-Cyclodextrin (βCD) was performed in water at room temperature (25 °C). The interactions of ABF with β-Cyclodextrin, and the stability constant (Ka) of the inclusion complex formed between them were investigated by using UV-visible spectroscopy. The biological effects of baclofen and the two analogues on alcohol dependence were studied in wistar rats. The anti-addiction effect of the analogues was tested by measuring the alcohol intake and the variation of the animal behaviour. The toxicity of the compounds was also analysed on liver injury markers. Results: The amino-3-phenylbutanoic acid (ABF1) and 3,4,5-trihydroxy-N-(methyl-2-acetate) benzamide (ABF2) were synthesized. The complexation of both analogues of baclofen (BF) with β-cyclodextrin (βCD) (ABF-βCD) was realized and confirmed by the stability constant of the inclusion complex (Ka) and Job’s method. The evaluation of anti-addiction activity in vivo showed that ABF1-βCD inhibits the consumption of alcohol at doses equivalent to those of baclofen. Both baclofen analogues have shown an anxiolytic effect. Regarding the toxicity of the two compounds, our results showed that ABF1-βCD has less toxic effect than baclofen; it reduces the activity of ALT and AST enzymes. Histologically, ABF1-βCD has no effect on the liver structure and has a protective effect against lesions alcohol-induced liver disease. Conclusion: Therefore, it can be suggested that ABF1 analogue combined with β-Cyclodextrin can be used as a treatment for alcohol dependence. Further clinical works are needed to confirm its effectiveness.
Catalytic Intermolecular Carboamination of Unactivated Alkenes via Directed Aminopalladation
Liu, Zhen,Wang, Yanyan,Wang, Zichen,Zeng, Tian,Liu, Peng,Engle, Keary M.
supporting information, p. 11261 - 11270 (2017/08/22)
An intermolecular 1,2-carboamination of unactivated alkenes proceeding via a Pd(II)/Pd(IV) catalytic cycle has been developed. To realize this transformation, a cleavable bidentate directing group is used to control the regioselectivity of aminopalladation and stabilize the resulting organopalladium(II) intermediate, such that oxidative addition to a carbon electrophile outcompetes potential β-hydride elimination. Under the optimized reaction conditions, a broad range of nitrogen nucleophiles and carbon electrophiles are compatible coupling partners in this reaction, affording moderate to high yields. The products of this reaction can be easily converted to free ?3-amino acids and ?3-lactams, both of which are common structural motifs found in drug molecules and bioactive compounds. Reaction kinetics and DFT calculations shed light on the mechanism of the reaction and explain empirically observed reactivity trends.
1078-21-3 Process route
-
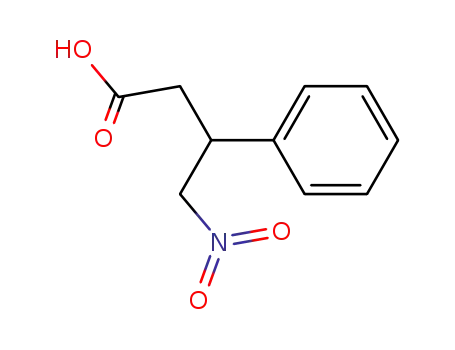
-
41441-41-2
4-nitro-3-phenyl butanoic acid

-
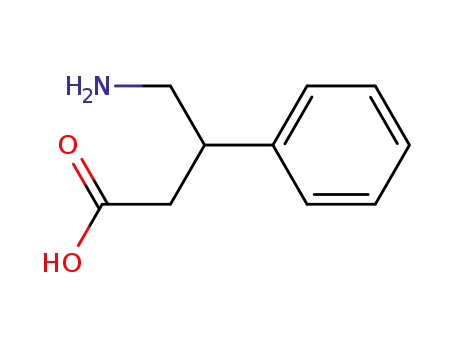
-
1078-21-3,35568-36-6,62596-63-8
4-amino-3-phenylbutanoic acid
| Conditions | Yield |
|---|---|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 60 ℃;
under 45004.5 Torr;
|
98% |
-
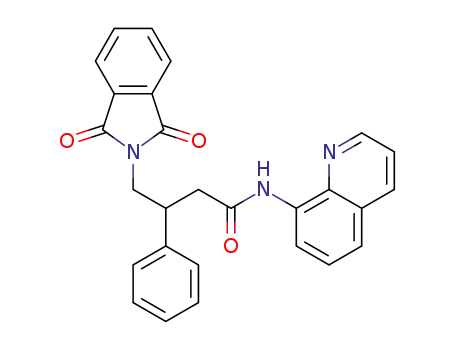
-
4-(1,3-dioxoisoindolin-2-yl)-3-phenyl-N-(quinolin-8-yl)butanamide

-

-
1078-21-3,35568-36-6,62596-63-8
4-amino-3-phenylbutanoic acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 130 ℃;
for 24h;
Sealed tube;
|
95% |
1078-21-3 Upstream products
-
52450-32-5
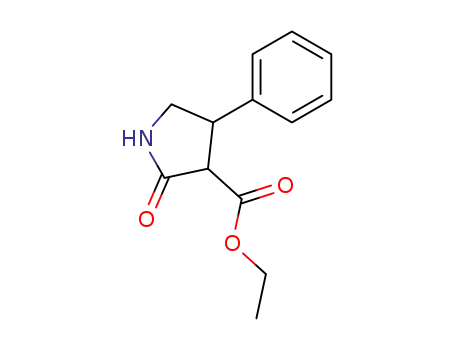
2-oxo-4-phenyl-3-ethoxycarbonylpyrrolidine
-
1198-97-6
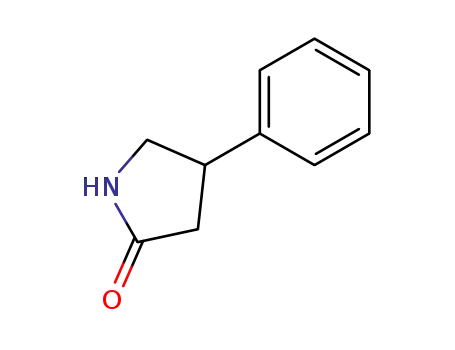
4-phenylpyrrolidin-2-one
-
14387-18-9
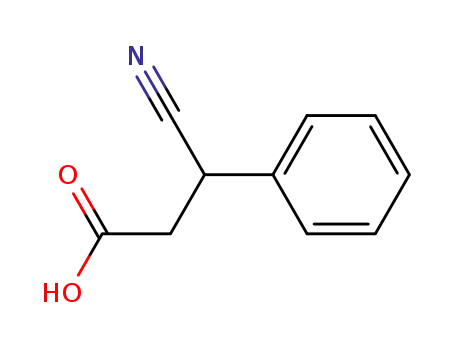
3-cyano-3-phenyl propionic acid
-
7647-01-0
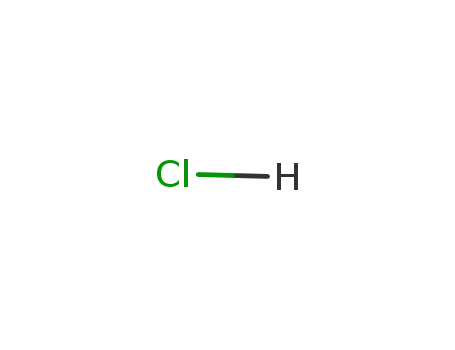
hydrogenchloride
1078-21-3 Downstream products
-
1078-21-3
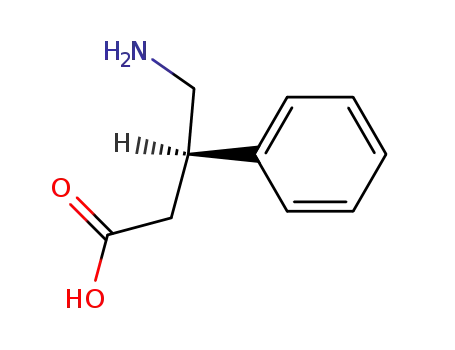
(R)-Phenibut
-
1078-21-3
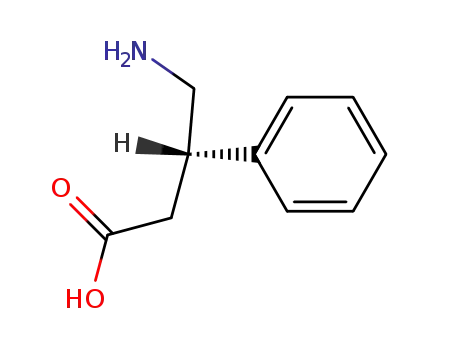
(S)-Phenibut
-
934269-53-1
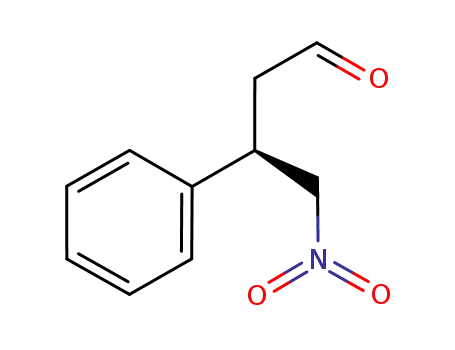
(3R)-4-nitro-3-phenylbutanal
-
189014-01-5
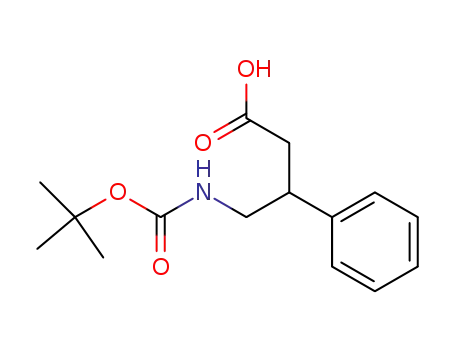
4-((tert-butoxycarbonyl)amino)-3-phenylbutanoic acid








