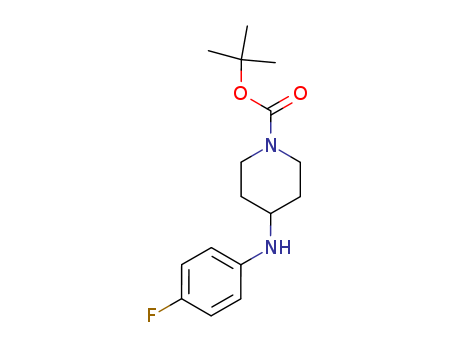
tert-butyl 4-(4-fluoroanilino)piperidine-1-carboxylate 288573-56-8
- CasNo:288573-56-8
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
288573-56-8 Properties
- Molecular Formula:C16H23 F N2 O2
- Molecular Weight:294.369
- Boiling Point:400.4±40.0 °C(Predicted)
- PKA:5.09±0.20(Predicted)
- PSA:41.57000
- Density:1.159±0.06 g/cm3(Predicted)
- LogP:3.64800
288573-56-8 Usage
Uses
1-BOC-4-(4-FLUORO-PHENYLAMINO)-PIPERIDINE is a heterocyclic derivative and can be used as a pharmaceutical intermediate.
288573-56-8 Relevant articles
Dual targeting of acetylcholinesterase and tau aggregation: Design, synthesis and evaluation of multifunctional deoxyvasicinone analogues for Alzheimer's disease
Manzoor, Shoaib,Gabr, Moustafa T.,Rasool, Bisma,Pal, Kavita,Hoda, Nasimul
, (2021/09/28)
Development of multitargeted ligands have demonstrated remarkable efficiency as potential therapeutics for Alzheimer's disease (AD). Herein, we reported a new series of deoxyvasicinone analogues as dual inhibitor of acetylcholinesterase (AChE) and tau aggregation that function as multitargeted ligands for AD. All the multitargeted ligands 11(a-j) and 15(a-g) were designed, synthesized, and validated by 1HNMR, 13CNMR and mass spectrometry. All the synthesized compounds 11(a-j) and 15(a-g) were screened for their ability to inhibit AChE, BACE1, amyloid fibrillation, α-syn aggregation, and tau aggregation. All the screened compounds possessed weak inhibition of BACE-1, Aβ42 and α-syn aggregation. However, several compounds were identified as potential hits in the AChE inhibitory screening assay and cellular tau aggregation screening. Among all compounds, 11f remarkably inhibited AChE activity and cellular tau oligomerization at single-dose screening (10 μM). Moreover, 11f displayed a half-maximal inhibitory concentration (IC50) value of 0.91 ± 0.05 μM and half-maximal effective concentration (EC50) value of 3.83 ± 0.51 μM for the inhibition of AChE and cellular tau oligomerization, respectively. In addition, the neuroprotective effect of 11f was determined in tau-expressing SH-SY5Y cells incubated with Aβ oligomers. These findings highlighted the potential of 11f to function as a multifunctional ligand for the development of promising anti-AD drugs.
TRICYCLIC COMPOUNDS
-
Paragraph 00282, (2017/08/01)
Provided herein are compounds and pharmaceutical compositions comprising said compounds that are useful for treating cancers or congenital diseases. Specific cancers and congenital disease includes those that are mediated by YAP/TAZ.
Triazinone compound and T-type calcium channel inhibitor
-
Page/Page column 82, (2016/08/29)
There is provided a novel triazinone compound that has an excellent T-type voltage-dependent calcium channel inhibitory activity and is specifically useful for treatment of pain. A compound of Formula (I), a tautomer of the compound, a pharmaceutically acceptable salt thereof, or a solvate thereof: where each substituent is defined in detail in the description or claims, for example R1 is H or C1-6 alkoxy, etc., each of L1 and L2 is independently a single bond or NR2, etc., L3 is C1-6 alkylene, etc., A is C6-14 aryl or 5 to 10-membered heteroaryl which is optionally substituted, etc., B is C3-11 cycloalkylene, etc., D is C6-14 aryl or 5 to 10-membered heteroaryl which is optionally substituted, etc.
HYDROXYQUINOXALINECARBOXAMIDE DERIVATIVE
-
Page/Page column 50, (2010/12/29)
The present invention provides a novel hydroxyquinoxaline carboxamide derivative that is useful for preventing and/or treating blood coagulation disorders. A compound represented by formula (i), or a pharmacologically acceptable salt thereof: wherein, each of R1 and R2 independently represents a group such as a hydrogen atom or a halogen atom; R3 represents a group such as a hydrogen atom; each of R4 and R5 independently represents a group such as a hydrogen atom, a halogen atom or a C1-4 alkyl group; each of R6 and R7 independently represents a hydrogen atom or a C1-4 alkyl group; X represents a group such as a C3-10 cycloalkyl group, C6-10 aryl group or a 5- to 10-membered heterocyclic group, which may be substituted with substituent(s) selected from substituent group α; Y represents a group such as -CO-, -O- or -NRa-, and Ra represents a group such as a hydrogen atom or a C1-4 alkyl group.
288573-56-8 Process route
-
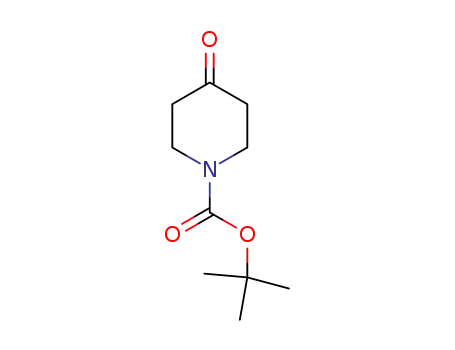
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
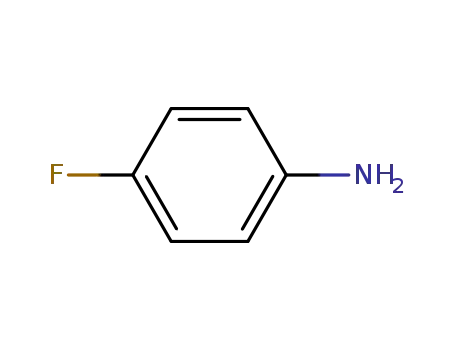
-
371-40-4
4-fluoroaniline

-
![1-(tert-Butoxycarbonyl)-4-[(4-fluorophenyl)amino]piperidine](/upload/2023/2/d75626e0-a7d0-4004-a6a2-7dccf9dc91b2.png)
-
288573-56-8
1-(tert-Butoxycarbonyl)-4-[(4-fluorophenyl)amino]piperidine
| Conditions | Yield |
|---|---|
|
With
sodium cyanoborohydride; acetic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 15h;
|
80% |
|
With
sodium tris(acetoxy)borohydride; acetic acid;
In
1,2-dichloro-ethane;
at 20 ℃;
for 18h;
|
75% |
|
With
sodium tris(acetoxy)borohydride; acetic acid;
In
1,2-dichloro-ethane;
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
1,2-dichloro-ethane;
at 20 ℃;
for 24h;
With
sodium tris(acetoxy)borohydride;
In
1,2-dichloro-ethane;
for 24h;
|
|
|
|
|
|
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
1,2-dichloro-ethane;
at 20 ℃;
for 24h;
With
sodium tris(acetoxy)borohydride;
In
1,2-dichloro-ethane;
at 20 ℃;
for 24h;
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
1,2-dichloro-ethane;
at 20 ℃;
for 24h;
With
sodium tetrahydroborate;
In
1,2-dichloro-ethane;
for 24h;
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
1,2-dichloro-ethane;
at 15 ℃;
for 0.166667h;
With
sodium tris(acetoxy)borohydride;
In
1,2-dichloro-ethane;
at 15 ℃;
for 2h;
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
1,2-dichloro-ethane;
at 20 ℃;
for 24h;
With
sodium tris(acetoxy)borohydride;
In
1,2-dichloro-ethane;
for 24h;
|
|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-fluoroaniline;
With
acetic acid;
In
methanol;
at 20 ℃;
With
methanol; sodium tetrahydroborate;
In
methanol;
at 20 ℃;
for 1h;
Cooling with ice;
|
-
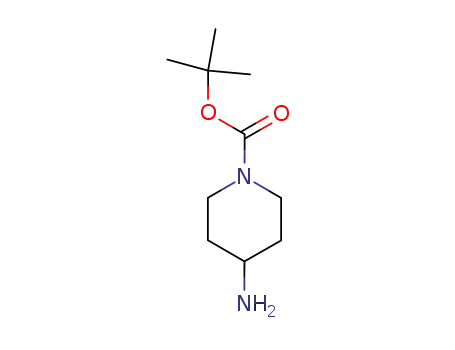
-
87120-72-7
1-(tert-butoxycarbonyl)-4-aminopiperidine

-
-
460-00-4
1-Bromo-4-fluorobenzene

-
![1-(tert-Butoxycarbonyl)-4-[(4-fluorophenyl)amino]piperidine](/upload/2023/2/d75626e0-a7d0-4004-a6a2-7dccf9dc91b2.png)
-
288573-56-8
1-(tert-Butoxycarbonyl)-4-[(4-fluorophenyl)amino]piperidine
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); DavePhos;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 140 ℃;
for 4h;
Inert atmosphere;
|
66% |
288573-56-8 Upstream products
-
79099-07-3

N-tert-butyloxycarbonylpiperidin-4-one
-
371-40-4
4-fluoroaniline
-
87120-72-7

1-(tert-butoxycarbonyl)-4-aminopiperidine
-
460-00-4
1-Bromo-4-fluorobenzene
288573-56-8 Downstream products
-
746649-08-1
4-[(4-fluoro-phenyl)-(3-methoxy-benzoyl)-amino]-piperidine-1-carboxylic acid tert-butyl ester
-
501674-31-3
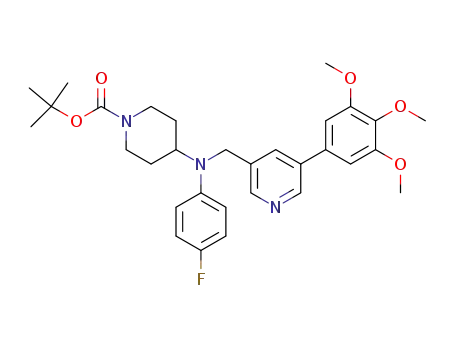
1-(tert-Butoxycarbonyl)-4-[N-(4-fluorophenyl)-N-[[3-(3,4,5-trimethoxyphenyl)pyridin-5-yl]methyl]amino]piperidine
-
501674-33-5
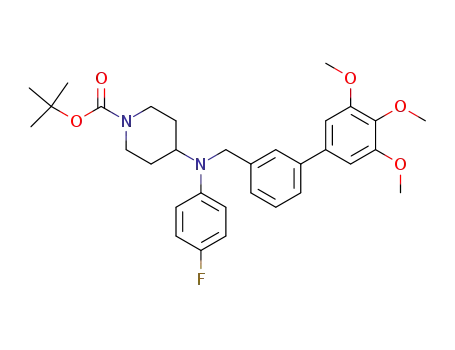
1-(tert-Butoxycarbonyl)-4-[N-(4-fluorophenyl)-N-[3-(3,4,5-trimethoxyphenyl)benzyl]amino]piperidine
-
1189550-02-4
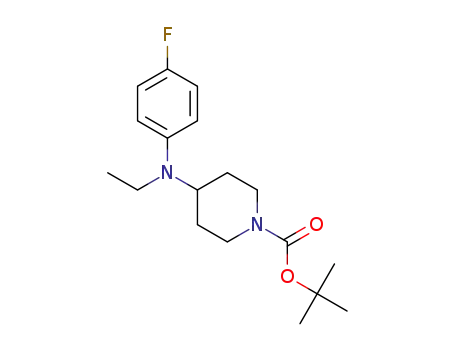
t-butyl 4-[(ethyl)(4-fluorophenyl)amino]piperidine-1-carboxylate








