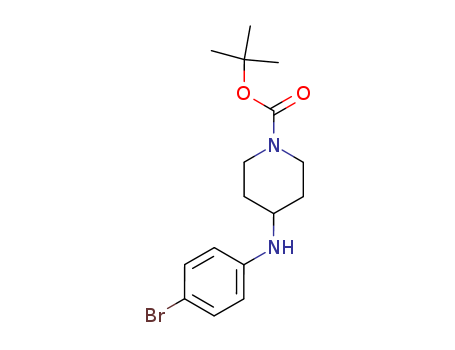
1-BOC-4-(4-BROMO-PHENYLAMINO)-PIPERIDIN 443998-65-0
- CasNo:443998-65-0
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
443998-65-0 Properties
- Molecular Formula:C16H23BrN2O2
- Molecular Weight:355.275
- Boiling Point:439.1±40.0 °C(Predicted)
- PKA:4.18±0.20(Predicted)
- PSA:41.57000
- Density:1.336±0.06 g/cm3(Predicted)
- LogP:4.27140
443998-65-0 Relevant articles
TRIAZOLE DERIVATIVES WITH ANTIFUNGAL ACTIVITY
-
Paragraph 00294, (2021/08/14)
Disclosed are compounds of the formula (I) and pharmaceutically acceptable salts thereof, wherein R1, R2, Q2, L1 and n are as defined herein. The compounds have antifungal properties and are useful in the treatment of fungal infections, including infections that are resistant to conventions anti-fungal agents. Q1 is selected from: (Formulae Ia, Ib, Ic, Id, Ie, If, Ig, Ih, Ii, Ij and Ik) wherein * indicates the point of attachment to L1.
Design and synthesis of pyridin-2-ylmethylaminopiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication
Skerlj, Renato,Bridger, Gary,Zhou, Yuanxi,Bourque, Elyse,McEachern, Ernest,Langille, Jonathan,Harwig, Curtis,Veale, Duane,Yang, Wen,Li, Tongshong,Zhu, Yongbao,Bey, Michael,Baird, Ian,Sartori, Michael,Metz, Markus,Mosi, Renee,Nelson, Kim,Bodart, Veronique,Wong, Rebecca,Fricker, Simon,Mac Farland, Ron,Huskens, Dana,Schols, Dominique
scheme or table, p. 6950 - 6954 (2012/01/13)
A series of CCR5 antagonists were optimized for potent inhibition of R5 HIV-1 replication in peripheral blood mononuclear cells. Compounds that met acceptable ADME criteria, selectivity, human plasma protein binding, potency shift in the presence of α-glycoprotein were evaluated in rat and dog pharmacokinetics.
Reduction of hERG inhibitory activity in the 4-piperidinyl urea series of H3 antagonists
Berlin, Michael,Lee, Yoon Joo,Boyce, Christopher W.,Wang, Yi,Aslanian, Robert,McCormick, Kevin D.,Sorota, Steve,Williams, Shirley M.,West Jr., Robert E.,Korfmacher, Walter
scheme or table, p. 2359 - 2364 (2010/08/20)
Structural features of the substituted 4-piperidinyl urea analogs 1, responsible for the H3 antagonist activity, have been identified. Structure-activity relationship of the H3 receptor affinity, hERG ion channel inhibitory activity and their separation is described. Preliminary pharmacokinetic evaluation of the compounds of the series is addressed.
Biaryl ureas as potent and orally efficacious melanin concentrating hormone receptor 1 antagonists for the treatment of obesity
Palani, Anandan,Shapiro, Sherry,McBriar, Mark D.,Clader, John W.,Greenlee, William J.,Spar, Brian,Kowalski, Timothy J.,Farley, Constance,Cook, John,Van Heek, Margaret,Weig, Blair,O'Neill, Kim,Graziano, Michael,Hawes, Brian
, p. 4746 - 4749 (2007/10/03)
Herein, we report a small molecule MCH-R1 antagonist which demonstrates oral efficacy in chronic rodent models. Substituted phenyl biaryl urea derivatives were synthesized and evaluated as MCH-R1 antagonists for the treatment of obesity. The structure-activity relationship studies in this series resulted in identification of urea 1 as a potent and selective MCH-R1 antagonist. Compound 1 exhibited oral efficacy in chronic (28 d) rodent models at 3-30 mpk showing significant reduction in food intake and weight gain relative to controls.
443998-65-0 Process route
-
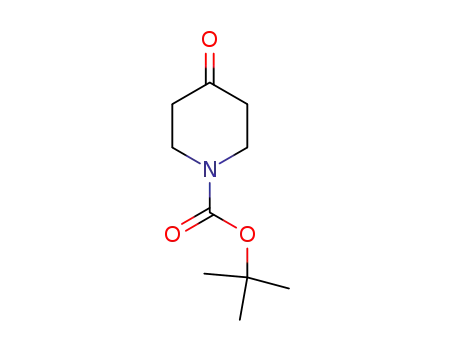
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
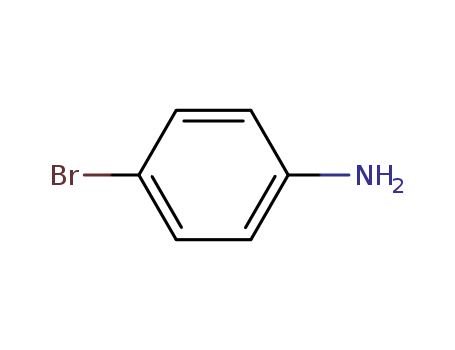
106-40-1
4-bromo-aniline

-
-
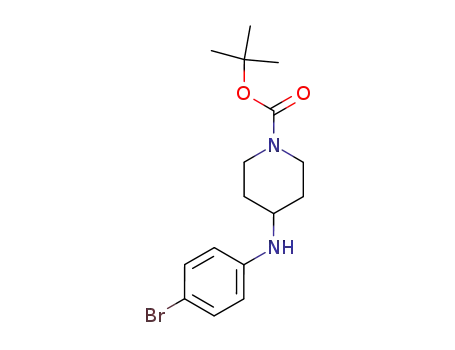
443998-65-0
4-(4-Bromophenyl)amino-1-(tert-butoxycarbonyl)piperidine
| Conditions | Yield |
|---|---|
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-bromo-aniline;
With
sodium tris(acetoxy)borohydride; acetic acid;
In
dichloromethane;
at 25 ℃;
for 17h;
With
sodium hydroxide;
In
dichloromethane; water;
|
72% |
|
N-tert-butyloxycarbonylpiperidin-4-one; 4-bromo-aniline;
With
titanium(IV) isopropylate;
at 20 ℃;
for 24h;
With
sodium cyanoborohydride;
In
methanol;
at 0 - 20 ℃;
for 5.33h;
|
49% |
|
|
|
|
|
|
|
With
titanium(IV) isopropylate; sodium cyanoborohydride;
In
dichloromethane;
|
|
|
With
sodium tris(acetoxy)borohydride; acetic acid;
In
1,2-dichloro-ethane;
|
|
|
With
sodium tris(acetoxy)borohydride; acetic acid;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|

-

-
443998-65-0
4-(4-Bromophenyl)amino-1-(tert-butoxycarbonyl)piperidine
| Conditions | Yield |
|---|---|
|
|
36% |
443998-65-0 Upstream products
-
79099-07-3

N-tert-butyloxycarbonylpiperidin-4-one
-
106-40-1

4-bromo-aniline
443998-65-0 Downstream products
-
862652-46-8
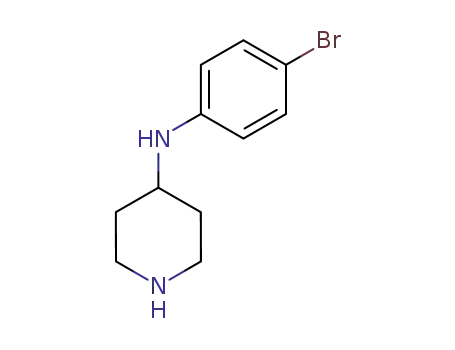
(4-bromo-phenyl)-piperidin-4-yl-amine
-
501674-12-0
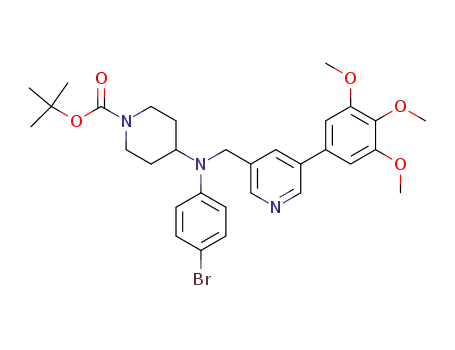
4-[N-(4-Bromophenyl)-N-[[3-(3,4,5-trimethoxyphenyl)pyridin-5-yl]methyl]amino]-1-(tert-butoxycarbonyl)piperidine
-
501674-14-2
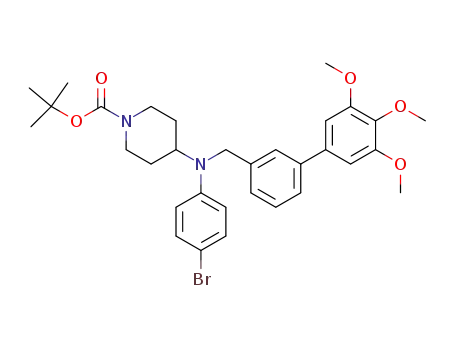
4-[N-(4-Bromophenyl)-N-[3-(3,4,5-trimethoxyphenyl)benzyl]amino]-1-(tert-butoxycarbonyl)piperidine
-
1534378-15-8
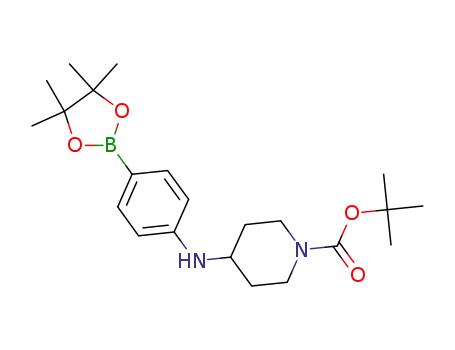
tert-butyl 4-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)amino)piperidine-1-carboxylate