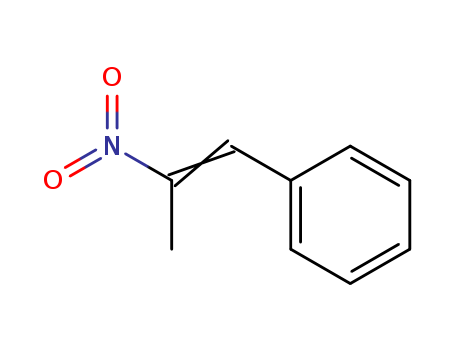
1-Phenyl-2-nitropropene/P2NP 705-60-2
- CasNo:705-60-2
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
705-60-2 Properties
- Molecular Formula:C9H9NO2
- Molecular Weight:163.176
- Appearance/Colour:Yellow crystalline powder
- Vapor Pressure:0.0172mmHg at 25°C
- Melting Point:64-67
- Refractive Index:1.586
- Boiling Point:263 °C at 760 mmHg
- Flash Point:115.7 °C
- PSA:45.82000
- Density:1.141 g/cm3
- LogP:2.84730
705-60-2 Usage
Chemical Properties
Yellow crystalline powder
Uses
1-phenyl-2-nitropropene is used in the production of pharmaceuticals, for instance, for drug Adderall, a drug used to treat ADHD and narcolepsy.
Application
(2-Nitropropenyl)benzene is a derivative of styrene (S687790), which exhibits herbicidal and antibacterial properties.
Synthesis Reference(s)
The Journal of Organic Chemistry, 15, p. 8, 1950 DOI: 10.1021/jo01147a002Tetrahedron Letters, 26, p. 1193, 1985 DOI: 10.1016/S0040-4039(00)98431-4
General Description
trans-β-Methyl-β-nitrostyrene (1-phenyl-2-nitropropene), a nitrostyrene derivative is an α,β-disubstituted nitroalkene. It has been synthesized by reacting benzaldehyde with nitroethane and butylamine. Spectroscopic analysis of 1-phenyl-2-nitropropene has been done using FT-IR, FT-Raman, NMR and UV.
InChI:InChI=1/C9H9NO2/c1-8(10(11)12)7-9-5-3-2-4-6-9/h2-7H,1H3/b8-7+
705-60-2 Relevant articles
Active Base Hybrid Organosilica Materials based on Pyrrolidine Builder Units for Fine Chemicals Production
Llopis, Sebastián,Velty, Alexandra,Díaz, Urbano
, p. 5012 - 5024 (2021/10/19)
The catalytic activity of “pyrrolidine type” fragments included or anchored in the mesoporous silica supports or polymeric frameworks have been fully reported for enantioselective transformation. Nevertheless, low attention was focused on their catalytic abilities to perform base-catalyzed reaction. Accordingly, hybrid materials including pyrrolidine fragments in the mesoporous silica supports were prepared following different synthesis methods, such as micellar and fluoride sol-gel routes in absence of structural directing agents. Their great catalytic performance was explored for various base-catalyzed reactions to the formation of C?C bond through Knoevenagel, Claisen-Schmidt and Henry condensations under microwave irradiation. The benefits of microwave irradiation combined with suitable catalytic properties of pyrrolidine hybrid materials with strong base sites and high accessibility to active centers, allowed carrying out successfully base-catalyzed condensation reactions for the production of fine chemicals. Moreover, the hybrid catalyst exhibited high selectivity and good stability over different catalytic cycles contributing to environmental sustainability.
Diindenopyrazines: Electron-Deficient Arenes
Brosius, Victor,Bunz, Uwe H. F.,Freudenberg, Jan,Hippchen, Nikolai,Rominger, Frank,Weigold, Svenja
supporting information, p. 10001 - 10005 (2021/06/07)
The syntheses, properties and application of the air-stable electron acceptors, diindenopyrazines 4 a–g are reported demonstrating the introduction of functional aryl groups in the 6- and 12-positions. The targets are accessible on the hundred milligram to gram scale. The structure of the aryl groups in 4 a–g modulates their solubility, redox potentials and optical properties. The introduction of electron-poor aryl groups to the electron-poor diindenopyrazine backbone reduces the electron affinity to ?4 eV, making the compounds attractive as n-semiconductors. A simple organic field-effect transistor of 4 e –without optimization– shows electron transport with a mobility of up to 0.037 cm2 V?1 s?1.
Dipolar HCP materials as alternatives to DMF solvent for azide-based synthesis
Bai, Rongxian,Gao, Feng,Gu, Yanlong,Li, Minghao
supporting information, p. 7499 - 7505 (2021/10/12)
Hypercrosslinked polymers HCP-DMF and HCP-DMF-SO3H containing abundant and flexible DMF moieties were designed and synthesized. Benefitting from the solvation microenvironment provided by the pseudo-DMF moities, the polar HCPs manifested outstanding performances in the conversions of NaN3 to benzylic azides and 1,2,3-triazoles in EtOH (95%), respectively, avoiding the use of risky DMF and improving the separation processes of the products.
Ionic-Liquid Controlled Nitration of Double Bond: Highly Selective Synthesis of Nitrostyrenes and Benzonitriles
Casiello, Michele,Caputo, Daniela,Fusco, Caterina,Cotugno, Pietro,Rizzi, Vito,Dell'Anna, Maria Michela,D'Accolti, Lucia,Nacci, Angelo
supporting information, p. 6012 - 6018 (2020/08/24)
Unprecedented in literature, the conversion of aryl alkenes into β-nitrostyrenes (2) or benzonitriles (3) with sodium nitrite can be governed by an appropriate choice of ionic liquid (IL) medium. A general trend was found for the selectivity of these processes, which depends on the nature of IL, with imidazolium-based ILs, such as [Bmim]Cl, that favor the C–H nitration leading to β-nitrostyrenes, while tetraalkylammonium-based ILs, such as TBAA, privilege the C=C bond cleavage affording benzonitriles. Besides a substrate scope, mechanistic hypotheses were provided on the origin of the different selectivity in the two kinds of ILs, based on their own tunable properties such as polarity, viscosity, and solvent cage effects.
705-60-2 Process route
-
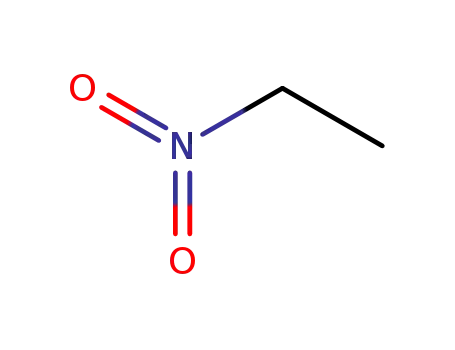
-
79-24-3
Nitroethane

-
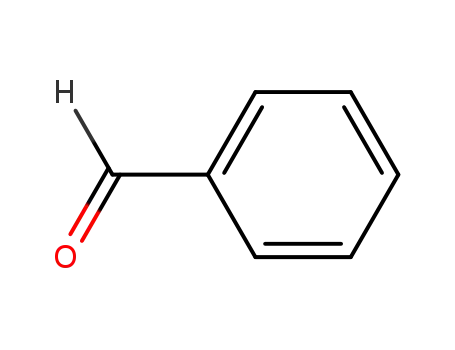
-
100-52-7
benzaldehyde

-
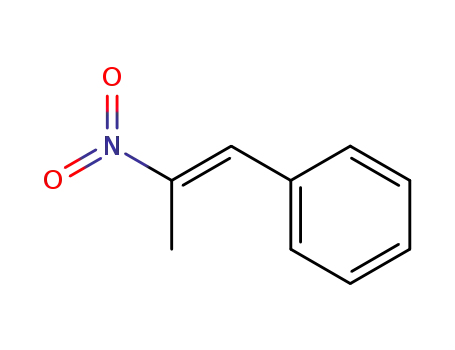
-
705-60-2,18315-84-9,58321-79-2
1-phenyl-2-nitroprop-1-ene

-

-
100-47-0
benzonitrile

-
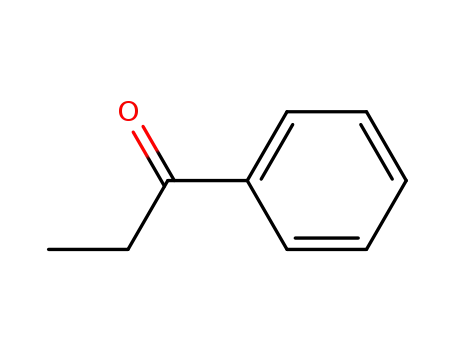
-
93-55-0
1-phenyl-propan-1-one

-
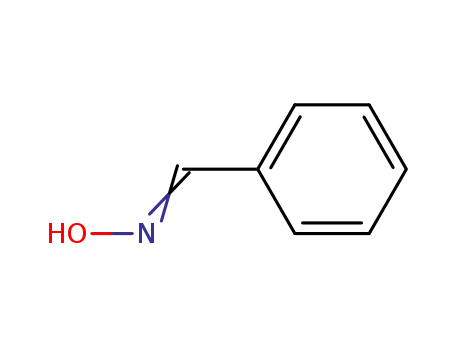
-
932-90-1
Benzaldoxime
| Conditions | Yield |
|---|---|
|
With
ammonium acetate;
In
acetic acid;
at 50 ℃;
for 3h;
under 7500600 Torr;
|
76% |
|
With
ammonium acetate;
In
acetic acid;
at 50 ℃;
for 3h;
under 7500600 Torr;
|
-
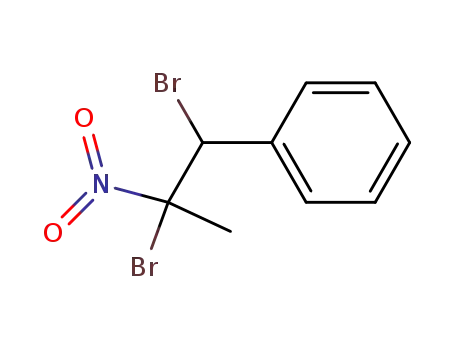
-
19419-13-7
(1,2-dibromo-2-nitro-propyl)-benzene

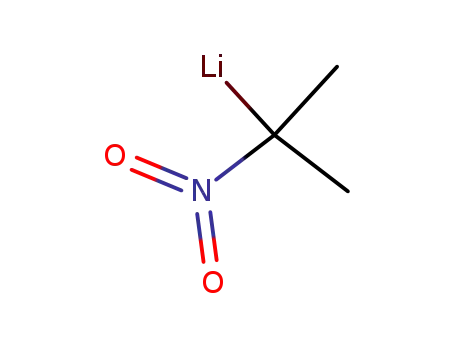
-
-
147225-22-7
2-lithio-2-nitropropane

-
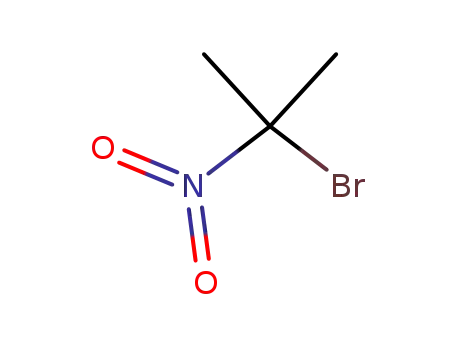
-
5447-97-2
2-bromo-2-nitropropane

-
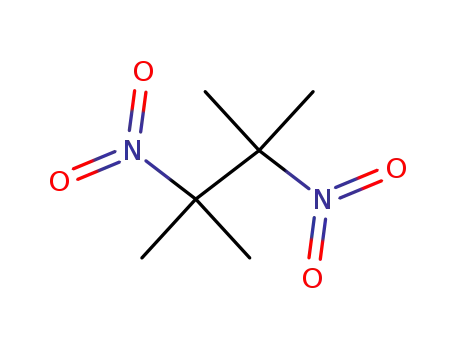
-
3964-18-9
2,3-dimethyl-2,3-dinitrobutane

-
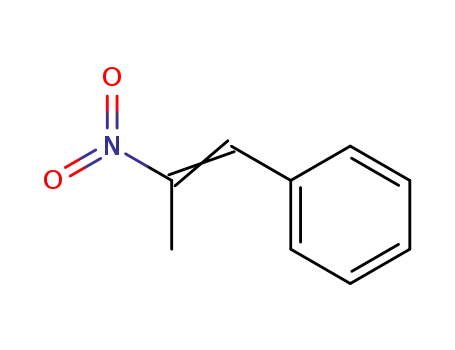
-
705-60-2
(2-nitroprop-1-enyl)benzene
| Conditions | Yield |
|---|---|
|
In
dimethyl sulfoxide;
Ambient temperature;
|
|
|
In
dimethyl sulfoxide;
for 0.166667h;
Mechanism;
Product distribution;
Ambient temperature;
also for other 1-aryl-1,2-dibromo-2-nitropropanes, also in the presence of p-dinitrobenzene or di-tert-butyl nitroxide;
|
705-60-2 Upstream products
-
79-24-3

Nitroethane
-
100-52-7

benzaldehyde
-
637-50-3
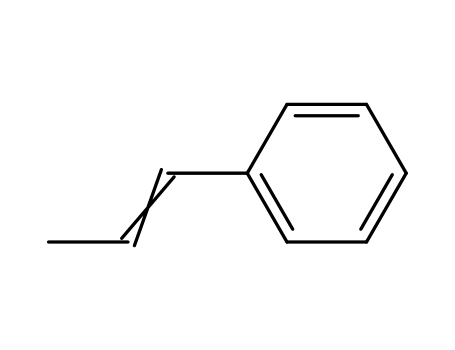
1-phenylpropene
-
26251-08-1
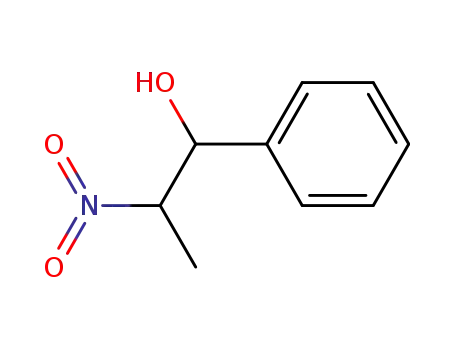
2-nitro-1-phenylpropan-1-ol
705-60-2 Downstream products
-
408314-96-5
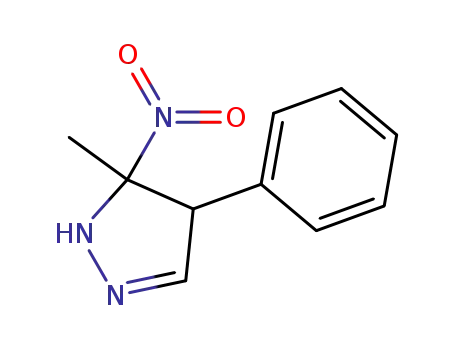
5-methyl-5-nitro-4-phenyl-4,5-dihydro-1H-pyrazole
-
28612-58-0
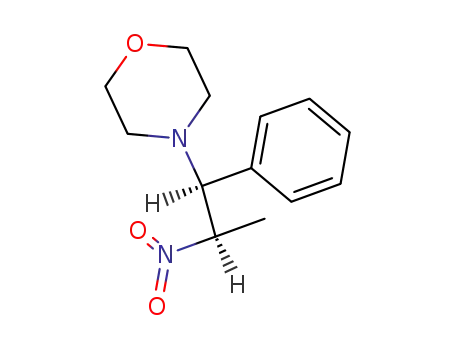
4-((1RS,2SR)-2-nitro-1-phenyl-propyl)-morpholine
-
28612-58-0
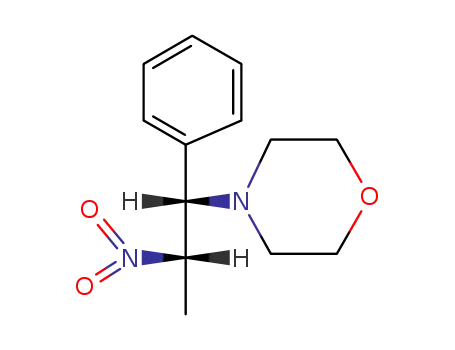
4-((1RS,2RS)-2-nitro-phenyl-propyl)-morpholine
-
93159-92-3
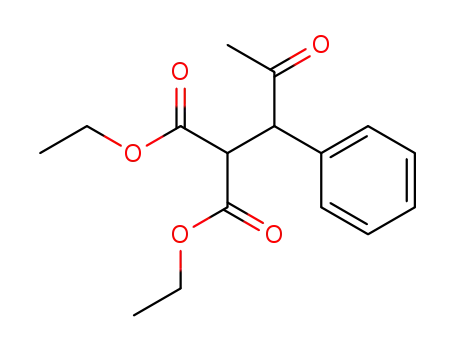
(2-oxo-1-phenyl-propyl)-malonic acid diethyl ester








