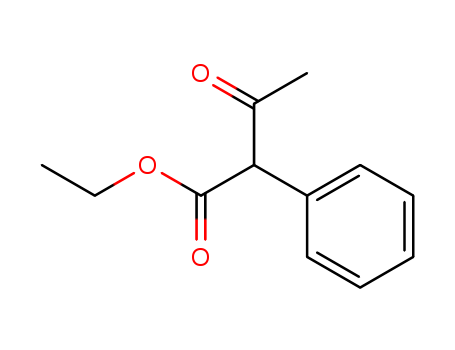
Ethyl 3-oxo-4-phenylbutanoate 5413-05-8
- CasNo:5413-05-8
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
5413-05-8 Properties
- Molecular Formula:C12H14O3
- Molecular Weight:206.241
- Vapor Pressure:0.00354mmHg at 25°C
- Melting Point:140-144 °C
- Refractive Index:1.5130
- Boiling Point:281.6 °C at 760 mmHg
- PKA:10.69±0.46(Predicted)
- Flash Point:119.2 °C
- PSA:43.37000
- Density:1.087 g/cm3
- LogP:1.92230
5413-05-8 Usage
Uses
Ethyl 2-Phenylacetoacetate is used in preparation of iridium polysubstituted quinoline diketonate complex and application as OLED.
InChI:InChI=1/C12H14O3/c1-3-15-12(14)11(9(2)13)10-7-5-4-6-8-10/h4-8,11H,3H2,1-2H3/t11-/m1/s1
5413-05-8 Relevant articles
Synthetic Scope of Br?nsted Acid-Catalyzed Reactions of Carbonyl Compounds and Ethyl Diazoacetate
Rahaman, Mizzanoor,Ali, M. Shahnawaz,Jahan, Khorshada,Hinz, Damon,Belayet, Jawad Bin,Majinski, Ryan,Hossain, M. Mahmun
, p. 6138 - 6147 (2021/05/06)
The comprehensive study of the reactions of carbonyl compounds and ethyl diazoacetate in the presence of a Br?nsted acid catalyst is described. In result, a broad range of 3-oxo-esters were synthesized from a variety of ketones and aliphatic aldehydes by 1,2-aryl/alkyl/hydride shift. Aryl-methyl ketones produced only aryl-migrated products, whereas other ketones yielded a mixture of products. For diaryl ketones, the identity of two inseparable migrated products was confirmed by two-dimensional NMR spectroscopy.
DMT1 Inhibitors Kill Cancer Stem Cells by Blocking Lysosomal Iron Translocation
Turcu, Andreea L.,Versini, Antoine,Khene, Nadjib,Gaillet, Christine,Ca?eque, Tatiana,Müller, Sebastian,Rodriguez, Rapha?l
supporting information, p. 7369 - 7373 (2020/06/02)
Cancer stem cells (CSC) constitute a cell subpopulation in solid tumors that is responsible for resistance to conventional chemotherapy, metastasis and cancer relapse. The natural product Salinomycin can selectively target this cell niche by directly interacting with lysosomal iron, taking advantage of upregulated iron homeostasis in CSC. Here, inhibitors of the divalent metal transporter 1 (DMT1) have been identified that selectively target CSC by blocking lysosomal iron translocation. This leads to lysosomal iron accumulation, production of reactive oxygen species and cell death with features of ferroptosis. DMT1 inhibitors selectively target CSC in primary cancer cells and circulating tumor cells, demonstrating the physiological relevance of this strategy. Taken together, this opens up opportunities to tackle unmet needs in anti-cancer therapy.
Substituted phenyl pyrazolone derivatives and preparation and application (by machine translation)
-
Paragraph 0034; 0037; 0038, (2019/01/08)
The present invention provides a substituted phenyl pyrazolone derivatives, in particular to a 2 - phenyl - pyrazoline - 3 - ketone compound and its pharmaceutically acceptable salt, solvate. The substituted acetic acid ethyl ester with sodium hydroxide in aqueous solution in the [...] reflux reaction, then in the palladium-carbon and hydrogen under the action of the hydrogenolysis benzyl, phenyl [...] obtained, with the bromochlorodifluoromethane alkane reaction to obtain the chloro, finally with the secondary amine on the condensation to obtain the target compound. The invention substituted phenyl pyrazoline compounds in vitro exhibits excellent capability of eliminating the free radicals, and have more strongly inhibit H3 receptor activity, exhibits excellent penetration of the blood brain barrier capacity, has a unique dual active, therefore, its for cerebral apoplexy, Alzheimer's disease, Parkinson's disease, progressive neurodegenerative diseases such as frozen sickness treatment has a unique clinical effect. The compound of the invention for treating central system system related disease and inflammatory disease application of the medicament. The formula structure is as follows: (by machine translation)
Silver-Catalyzed Olefination of Acetals and Ketals with Diazoesters to β-Alkoxyacrylates
Li, Jiawen,Qian, Bo,Huang, Hanmin
, p. 7090 - 7094 (2018/11/23)
The first silver-catalyzed reaction of acetals or ketals with diazoesters leading to trisubstituted or tetrasubstituted β-alkoxyacrylates is now reported. A broad range of acetals and ketals bearing different substituents is compatible with this protocol and thus provides an attractive approach for the synthesis of complex β-alkoxyacrylates. The power of this method was further demonstrated by the successful synthesis of picoxystrobin, which is one of the most popular agricultural fungicides commercialized by Dupont.
5413-05-8 Process route
-
-
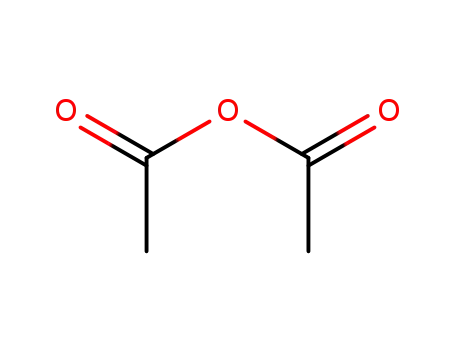
108-24-7
acetic anhydride

-
-
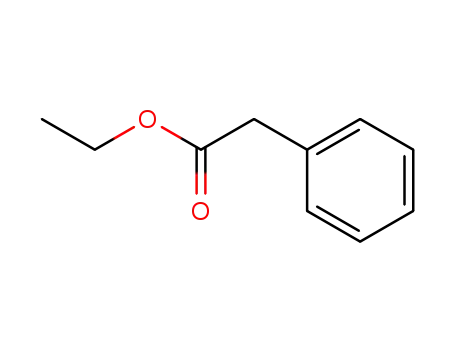
101-97-3
Ethyl 2-phenylethanoate

-
-
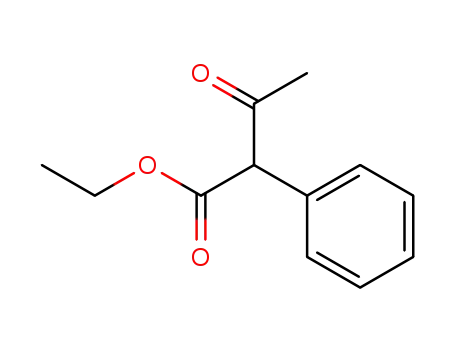
5413-05-8
ethyl 2-phenylacetoacetate
| Conditions | Yield |
|---|---|
|
Ethyl 2-phenylethanoate;
With
sodium hydride;
In
tetrahydrofuran;
at 60 ℃;
for 0.5h;
Cooling with ice;
acetic anhydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1h;
|
61% |
|
Ethyl 2-phenylethanoate;
With
sodium hydride;
In
tetrahydrofuran;
at 60 ℃;
for 0.5h;
acetic anhydride;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
51% |
|
Ethyl 2-phenylethanoate;
With
sodium hydride;
In
tetrahydrofuran;
at 60 ℃;
for 0.5h;
acetic anhydride;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
Further stages.;
|
51% |
|
Ethyl 2-phenylethanoate;
With
sodium hydride;
In
tetrahydrofuran;
at 60 ℃;
for 0.5h;
Inert atmosphere;
acetic anhydride;
In
tetrahydrofuran;
at 0 ℃;
|
-
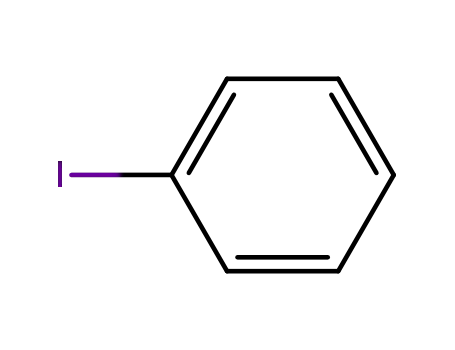
-
591-50-4
iodobenzene

-
-
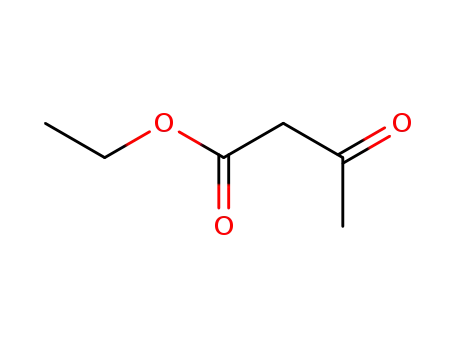
141-97-9
ethyl acetoacetate

-

-
5413-05-8
ethyl 2-phenylacetoacetate

-

-
101-97-3
Ethyl 2-phenylethanoate
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
copper(l) iodide;
In
dimethyl sulfoxide;
at 80 ℃;
for 20h;
|
89% 9% |
|
With
potassium phosphate;
copper(l) iodide;
In
dimethyl sulfoxide;
at 80 ℃;
for 20h;
|
26% 20% |
5413-05-8 Upstream products
-
17097-90-4
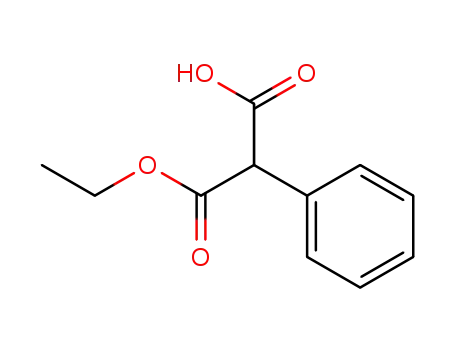
2-phenylmalonic acid monoethyl ester
-
920-39-8
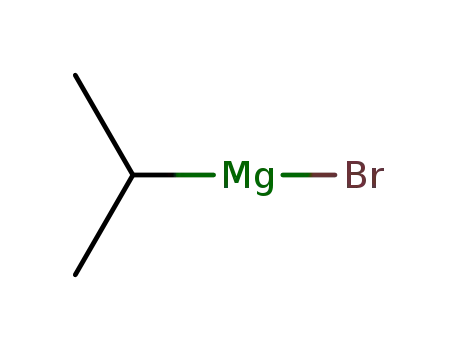
isopropylmagnesium bromide
-
141-78-6
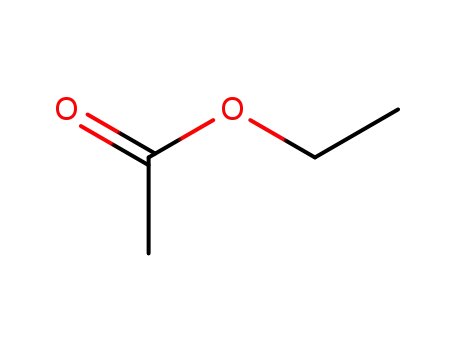
ethyl acetate
-
101-97-3

Ethyl 2-phenylethanoate
5413-05-8 Downstream products
-
861353-04-0
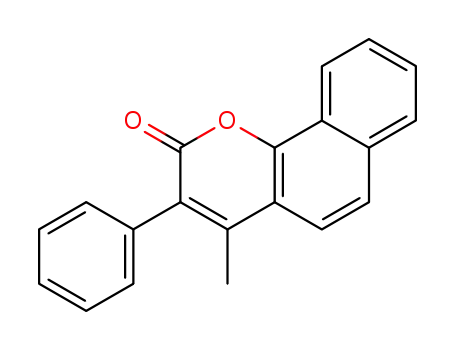
4-methyl-3-phenyl-benzo[h]chromen-2-one
-
52328-57-1
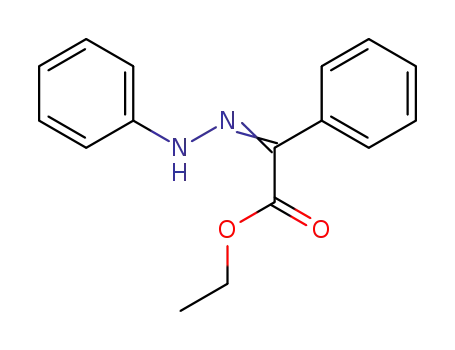
ethyl phenylhydrazono(phenyl)acetate
-
64754-67-2
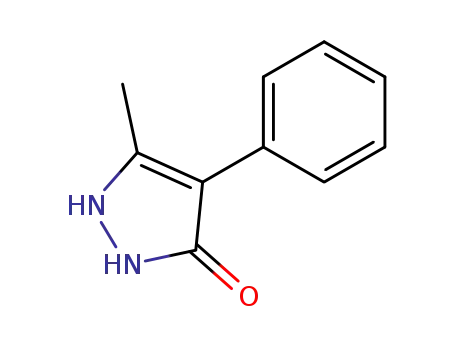
5-methyl-4-phenyl-1H-pyrazol-3(2H)-one
-
18648-91-4
methyl-5 phenyl-4 dithiole-1,2 thione-3








