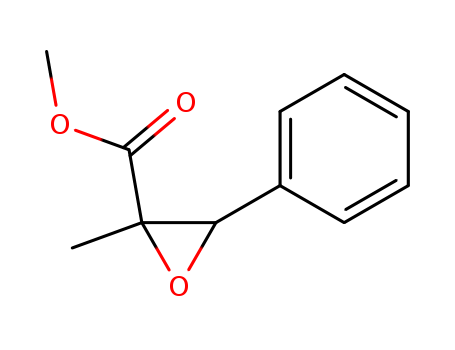
BMK methyl glycidate 80532-66-7
- CasNo:80532-66-7
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
80532-66-7 Properties
- Molecular Formula:C11H12O3
- Molecular Weight:192.214
- Boiling Point:73-74 °C(Press: 0.2 Torr)
- Density:1.168±0.06 g/cm3(Predicted)
80532-66-7 Usage
Description
BMK methyl glycidate is an analytical reference standard categorized as a precursor in the synthesis of phenylacetone.This product is intended for research and forensic applications.
Description
BMK methyl glycidate (Item No. 9002714) is an analytical reference standard categorized as a precursor in the synthesis of phenylacetone (Item Nos. 16103 | 16444). This product is intended for research and forensic applications.
Uses
2‐methyl-3-phenyloxirane-2- carboxylic acid (BMK glycidic acid) is substances that is immediate precursors of amphetamines and that is frequently used for the illicit manufacture of amphetamines.
80532-66-7 Relevant articles
Oxiranyl remote anions from epoxy cinnamates and their application towards the synthesis of α,β-epoxy-γ-butyrolactones
Sermmai, Patpanat,Ruangsupapichat, Nopporn,Thongpanchang, Tienthong
supporting information, (2020/11/19)
A series of α,β-epoxy-γ-butyrolactones were synthesized in moderate yields via oxiranyl remote anions derived from epoxy cinnamate esters. The key synthetic step involved deprotonation of the β-position of α,β-epoxy cinnamate derivatives where the generated β-anion was stabilized by remote chelation from an ester group. The substitution reaction of the anion with a variety of ketones, followed by cyclization, readily furnished the desired substituted α,β-epoxy-γ-butyrolactones.
Development of a robust and practical process for the Darzens condensation and α,β-epoxide rearrangement: Scope and limitations of the methodology
Zimbron, Jeremy Malcolm,Seeger-Weibel, Manuela,Hirt, Hans,Gallou, Fabrice
, p. 1221 - 1226 (2008/12/22)
A practical and robust process for the Darzens condensation of substituted benzaldehydes and subsequent α,β-epoxy rearrangement is reported. The process developed is both amenable to large scale and parallel synthesis. While electron-poor benzaldehydes gave mixtures of aryl ketones and 2-substituted aryl ketones in mediocre to low yields, electron-rich benzaldehydes were found to react in high yields with complete regioselectivity to form 2-substituted aryl ketones. Georg Thieme Verlag Stuttgart.
Ligand exchange reaction of sulfoxides in organic synthesis: A novel method for generation of magnesium enolates and its application to synthesis of α-halocarboxylic acid derivatives and α-haloaldehydes
Satoh,Kitoh,Onda,Takano,Yamakawa
, p. 4957 - 4972 (2007/10/02)
A new method for synthesis of α-halo(Cl, F)carboxylic acid derivatives and α-haloaldehydes is described. α-Halo-α-sulfinyl carboxylic acid, esters, and α-halo-α-sulfinyl aldehydes were easily prepared from aryl 1-haloalkyl sulfoxides and alkyl chloroformate and ethyl formate, respectively, in good yields. α-Chloro-α-sulfinyl amides were synthesized from (p-tolylthio)acetic acid. Ligand exchange reaction of the sulfinyl group of these acids, esters, amides, and aldehydes with ethylmagnesium bromide gave the magnesium enolates, which were treated with water to give α-halocarboxylic acid derivatives and α-chloroaldehydes in good yields. The magnesium enolates derived from the α-chloro-α-sulfinyl acid derivatives were trapped with carbonyl compounds to afford the adducts, which were transformed to α,β-epoxy carboxylic acid derivatives. Thermal elimination of the sulfinyl group in the α-halo-α-sulfinyl acid derivatives and the α-halo-sulfinyl aldehydes gave α-halo-α,β-unsaturated carboxylic acid derivatives and α-halo-α,β-unsaturated aldehydes in high yields.
MASS SPECTROMETRIC DECOMPOSITION OF β-PHENYLOXIRANECARBOXYLIC ESTERS
Anisimova, O. S.,Chistyakov, V. V.,Bokanov, A. I.,Shvedov, V. I.,Sheinker, Yu. N.
, p. 1058 - 1063 (2007/10/02)
The electron-impact mass spectra of the E- and Z-isomers of the α,β-methyl substituted esters of β-phenyloxiranecarboxylic acids have been studied.
80532-66-7 Process route
-
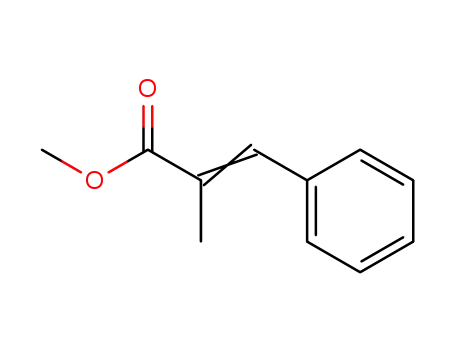
-
21370-57-0,22946-43-6,25692-59-5
methyl 2-methyl-3-phenylacrylate

-
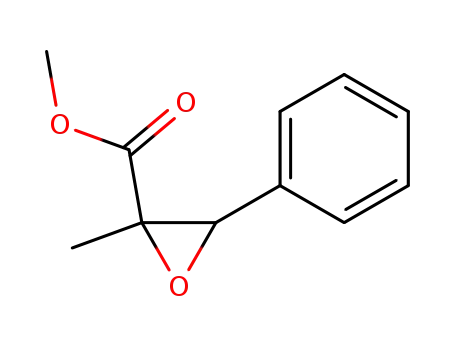
-
80532-66-7
methyl α,β-epoxy-α-methylcinnamate
| Conditions | Yield |
|---|---|
|
With
3-chloro-benzenecarboperoxoic acid;
In
tetrachloromethane;
for 3h;
Reflux;
|
67% |
-
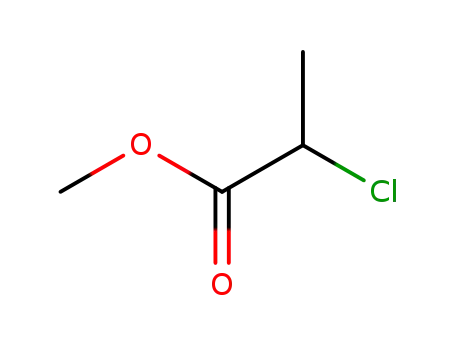
-
17639-93-9
2-chloro-propionic acid methyl ester

-
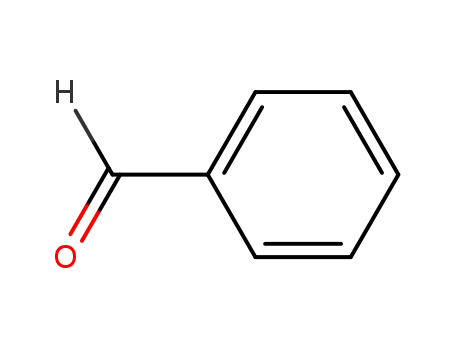
-
100-52-7
benzaldehyde

-

-
80532-66-7
methyl α,β-epoxy-α-methylcinnamate
| Conditions | Yield |
|---|---|
|
With
N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate;
In
N,N-dimethyl-formamide;
1.) 20 deg C, 72 h, 2.) 40 deg C, 24 h;
|
80% |
|
With
sodium methylate;
In
methanol; toluene;
at 0 - 20 ℃;
|
|
|
With
ethanol; sodium ethanolate;
|
80532-66-7 Upstream products
-
17639-93-9

2-chloro-propionic acid methyl ester
-
100-52-7

benzaldehyde
-
186581-53-3

diazomethane
-
25547-51-7
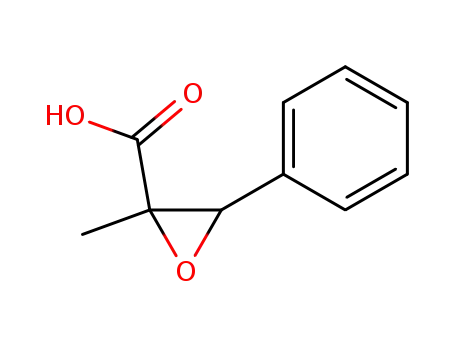
trans-2-methyl-3-phenyloxiranecarboxylic acid
80532-66-7 Downstream products
-
103-79-7
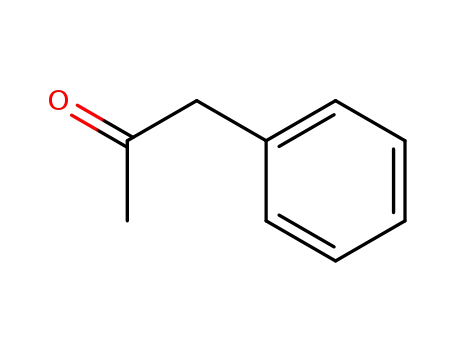
1-phenyl-acetone
-
95952-73-1
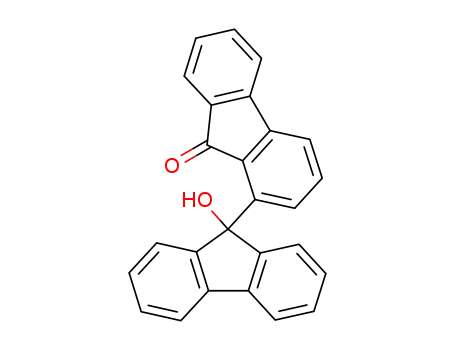
9'-hydroxy-9H,9'H-[1,9'-bifluoren]-9-one








