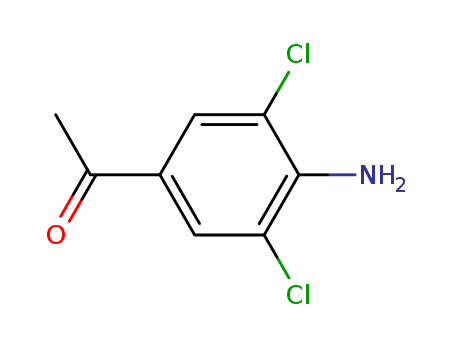
4-Amino-3,5-dichloroacetophenone 37148-48-4
- CasNo:37148-48-4
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
37148-48-4 Properties
- Molecular Formula:C8H7Cl2NO
- Molecular Weight:204.056
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:4.1E-05mmHg at 25°C
- Melting Point:162-166 °C(lit.)
- Refractive Index:1.599
- Boiling Point:351.5 °C at 760 mmHg
- PKA:-1.72±0.10(Predicted)
- Flash Point:166.4 °C
- PSA:43.09000
- Density:1.386 g/cm3
- LogP:3.35940
37148-48-4 Usage
Description
4-Amino-3,5-dichloroacetophenone is an intermediate in the synthesis of the antitussive and antiasthmatic drug gramtin.
Storage
4-Amino-3,5-dichloroacetophenone should be stored in a cool, dry place in a small, well filled, well-closed container, protected from light. When a partially filled container is used, the air should be replaced by nitrogen or another inert gas. 4-Amino-3,5-dichloroacetophenone oxidizes on exposure to air, resulting in an increase in the peroxide value.
Chemical Properties
4-Amino-3,5-dichloroacetophenone is a white to light yellow crystal powder. Insoluble in cold water, slightly soluble in hot water.
Uses
4-Amino-3,5-dichloroacetophenone is used to synthesize the drug Clenbuterol. Clenbuterol is a steroid-like chemical that was initially developed to treat asthma in horses, working by relaxing the airways in the animals'lungs.
Preparation
4-Amino-3,5-dichloroacetophenone was synthesized by chlorination of 4-aminoacetophenone. Add 4-aminoacetophenone and acetic acid (80%) into the reaction pot, stir and dissolve, quickly add chlorine-containing glacial acetic acid solution, the temperature of the addition is 5°C. After the addition is completed, immediately put into ice water to precipitate, filter, and wash with water to obtain the crude product. Recrystallize with ethanol to obtain the finished product of 4-Amino-3,5-dichloroacetophenone.
Application
4-Amino-3,5-dichloroacetophenone is a compound useful in organic synthesis.
InChI:InChI=1/C8H7Cl2NO/c1-4(12)5-2-6(9)8(11)7(10)3-5/h2-3H,11H2,1H3
37148-48-4 Relevant articles
Phenylethanolamine β receptor agonist synthetic method
-
Paragraph 0038-0041, (2019/07/04)
The invention discloses a phenylethanolamine β receptor agonist synthetic method, comprises the following steps: S1: the 4 - amino acetophenone dissolved in an organic solvent, with the electrophilic reagent occurs on the benzene ring substituted halogenated reaction, generating [...] intermediate; [...] intermediates in organic solvent or in water, under the catalysis of the metal catalyst with the cyanide reagent undergo nucleophilic substitution reaction, generating phenyl ketone intermediate; S2: phenyl ketone intermediates in organic solvent, with the copper bromide generating carbonyl α bromo reaction to produce α - bromoacetophenone intermediates; S3: α - bromoacetophenone intermediates in organic solvent with tert-butyl amine or isopropylamine reaction intermediates acetophenone amines; S4: acetophenone amine intermediates in organic solvent, with the reduction hydrogenation reagent react to generate the phenylethanolamine β receptor agonists; synthetic method of this invention a simple and highly efficient and cheap and easy to obtain, atom utilization is high, the synthetic product chemical purity is greater than 99%, to meet the detection requirements of the food safety.
3,5-dichloro-4-bromoisoquinoline derivative, and preparation method and application thereof
-
Paragraph 0036-0037, (2019/10/04)
The invention specifically relates to a novel compound, i.e., a 3,5-dichloro-4-bromoisoquinoline derivative, and a preparation method and application thereof, belonging to the technical field of pharmaceutical synthesis. 3,5-dichloro-4-bromoisoquinoline and the derivative thereof are obtained by ring closure of 3,5-dichloro-4-bromobenzene haloethylamine through a Friedel-Crafts alkylation reaction. The 3,5-dichloro-4-bromoisoquinoline and the derivative can be used for synthesizing the important intermediate 3,5-dichloro-4-carboxylisoquinoline of Lifitegrast. According to a technical scheme in the invention, low-cost and well-supplied 2,6-dichloroaniline is used as a starting material; and the preparation method comprises a plurality steps of reactions with mild conditions, mature operation and yield of 85% or more, and each step of reaction has passed pilot-scale test, so the feasibility of industrial conversion of the method is very high and overall production cost can be greatly reduced.
Stable isotope labeling Clenproperol compound and synthesis method thereof
-
Paragraph 0168-0169, (2018/02/04)
The invention relates to a stable isotope labeling Clenproperol compound and a synthesis method thereof. The method comprises the following steps of (1) using stable isotope labeling 2,6-dichloroaniline as raw materials to generate stable isotope labeling 3,5-dichloro-4-aminoacetophenone through Friedel-Crafts acyl reaction; (2) performing bromination on the stable isotope labeling 3,5-dichloro-4-aminoacetophenone to obtain stable isotope labeling 3,5-dichloro-4-amino-alpha-bromo acetophenone; (3) enabling the stable isotope labeling 3,5-dichloro-4-amino-alpha-bromo acetophenone and isopropylamine to take a reaction for obtaining stable isotope labeling 1-(4-amino-3,5-dichlone)-2-isopropyl amino ethyl ketone; (4) enabling the stable isotope labeling 1-(4-amino-3,5-dichlone)-2-isopropyl amino ethyl ketone and a reducing agent to take a reaction to generate stable isotope labeling Clenproperol. The stable isotope labeling Clenproperol compound and the synthesis method have the advantages that the process route is simple; the synthesis is easy; the stable isotope atom utilization rate is high; the obtained product can be easily separated and purified; the chemical purity and the fractional isotopic abudance are 99 percent or higher; the trace detection requirements in the food safety field can be sufficiently met.
37148-48-4 Process route
-
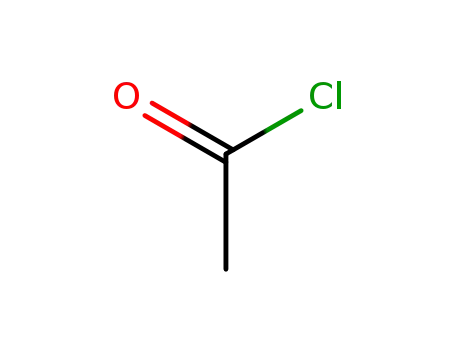
-
75-36-5
acetyl chloride

-
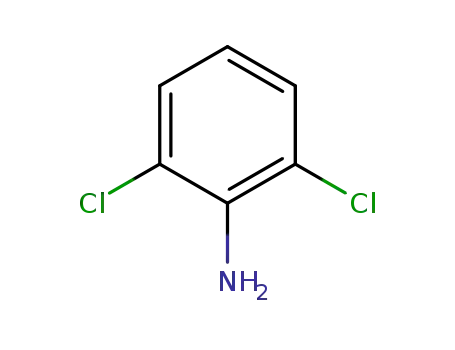
-
608-31-1
2,6-Dichloroaniline

-
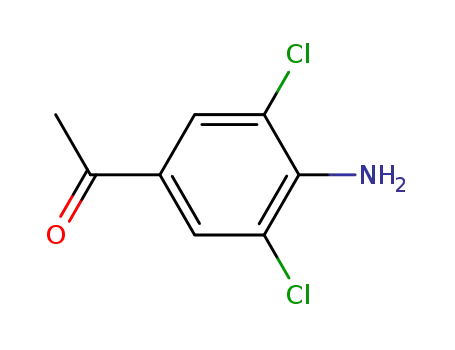
-
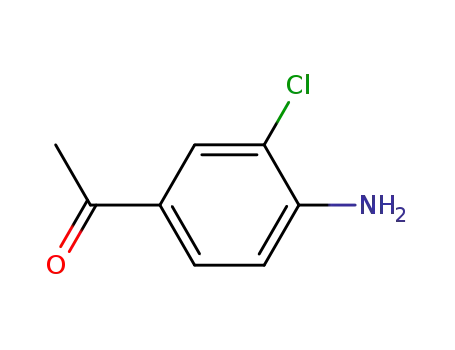
37148-48-4
1-(4-amino-3,5-dichlorophenyl)-1-ethanone
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride;
In
chloroform;
at 0 - 20 ℃;
for 6h;
|
88.6% |
-
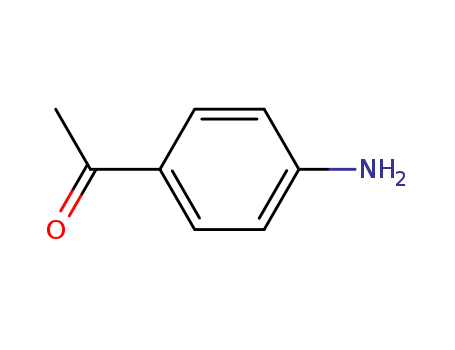
-
99-92-3
p-aminobenzophenone

-
-
6953-83-9
1-(4-amino-3-chlorophenyl)ethan-1-one

-

-
37148-48-4
1-(4-amino-3,5-dichlorophenyl)-1-ethanone
| Conditions | Yield |
|---|---|
|
With
N-chloro-succinimide;
In
acetonitrile;
at 20 ℃;
for 3.5h;
|
80% 18% |
|
With
N-chloro-succinimide;
In
acetonitrile;
at 20 ℃;
for 4.5h;
|
20% 45% |
37148-48-4 Upstream products
-
99-92-3

p-aminobenzophenone
-
7782-50-5
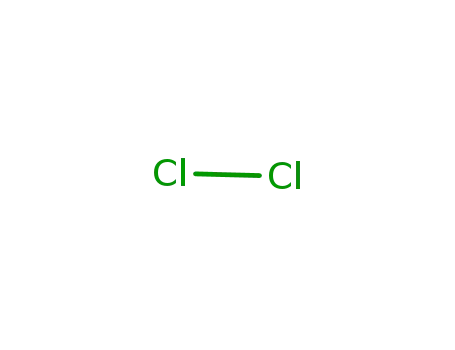
chlorine
-
64-19-7
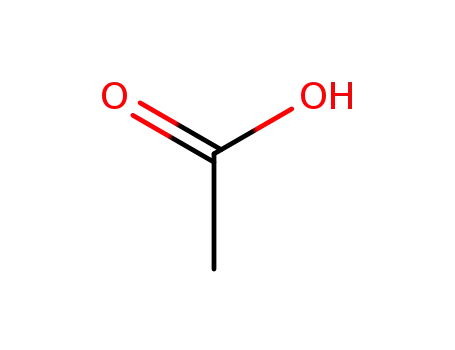
acetic acid
-
75-36-5

acetyl chloride
37148-48-4 Downstream products
-
60677-46-5
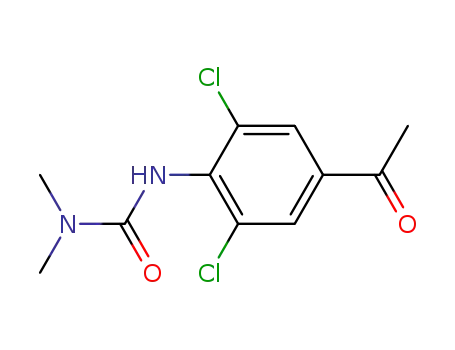
3,5-Dichlor-4-N,N-dimethylcarbamoylaminoacetophenon
-
60677-48-7
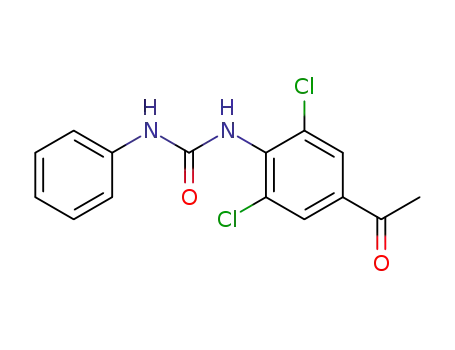
3,5-dichloro-4-phenylcarbamoylaminoacetophenone
-
349534-87-8
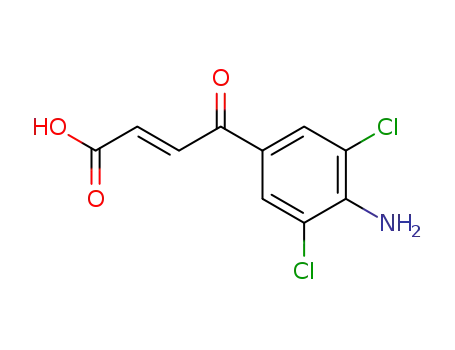
(E)-4-amino-3,5-dichloro-γ-oxo-benzenebutenoic acid
-
109346-95-4
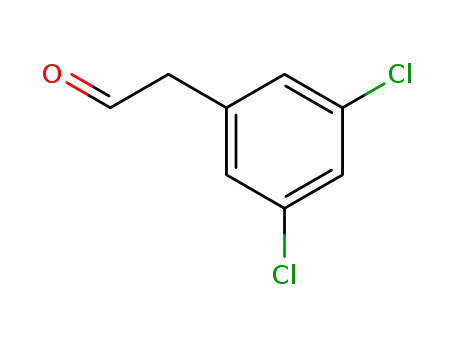
2-(3,5-dichlorophenyl)acetaldehyde








