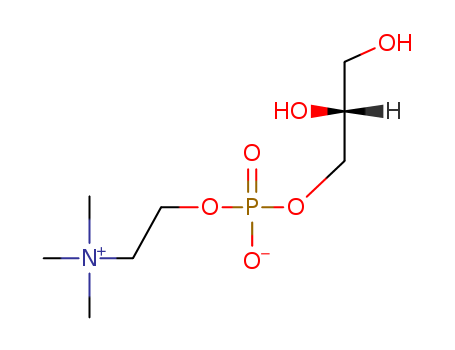
Choline glycerophosphate 28319-77-9
- CasNo:28319-77-9
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
28319-77-9 Properties
- Molecular Formula:C8H20NO6P
- Molecular Weight:257.224
- Appearance/Colour:White waxy solid
- Vapor Pressure:0Pa at 25℃
- Melting Point:-98oC
- Boiling Point:480℃[at 101 325 Pa]
- Flash Point:11 °C
- PSA:108.86000
- LogP:-0.38240
28319-77-9 Usage
Description
Choline alfoscerate is a nootropic reportedly effective in the treatment of age-associated memory impairment. In man, it decreased and prevented scopolamine-induced amnesia. In vitro studies suggest that choline alfoscerate acts indirectly on cholinergic transmission by elevating the synthesis of acetylcholine.
Chemical Properties
White waxy solid
Originator
Sandoz; Italfarmaco (Italy)
Uses
sn-Glycero-3-phosphocholine (Choline Alfoscerate) is a phospholipid; precursor in choline biosynthesis. sn-Glycero-3-phosphocholine is an intermediate in catabolic pathway of phosphatidylcholine. sn-Glycero-3-phosphocholine is used as an Nootropic.
Uses
L-α-Glycerophosphorylcholine has been used to rescue choline auxotrophy. It has also been used for the synthesis of glycerophospholipids.
Definition
ChEBI: A member of the class of phosphocholines that is the choline ester of sn-glycero-3-phosphate. It is one of the major osmolyte in the renal medullary cells.
Brand name
Delecit
General Description
L-α-Glycerophosphorylcholine is a phospholipid, which is a derivative of phosphatidylcholine.
Flammability and Explosibility
Notclassified
Biochem/physiol Actions
Increases inositol phosphate formation.
InChI:InChI=1/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/t8-/m1/s1
28319-77-9 Relevant articles
-
Baer,Kates
, p. 1394,1396 (1948)
-
Preparation method of glycerophosphocholine
-
Paragraph 0028-0032; 0033-0037; 0038-0042; 0043-0047, (2021/09/08)
The invention discloses a preparation method of glycerophosphocholine, which comprises the following steps: 1, dissolving lecithin in an alcohol solvent, then adding inorganic base or organic base for hydrolysis reaction to obtain a reaction solution, filtering, adding acid into the filtrate to adjust the pH value, and drying the alcohol solvent by distillation under reduced pressure to obtain a compound (I); 2, dissolving the compound (I) in water, and adding an extraction solvent for extraction to obtain a compound (II); and 3, carrying out active carbon decoloration on the compound (II) through a water phase, directly stirring by using mixed anion-cation resin, drying by distillation, and adding a recrystallization solvent to carry out recrystallization reaction to obtain refined glycerophosphocholine. According to the invention, lecithin is hydrolyzed in an alcohol solvent by using an inorganic weak base through a chemical hydrolysis method, and glycerophosphocholine which is very high in purity and reaches a medicinal level is obtained through a series of simple post-treatment processes, and the production process is simple, easy to control, low in material cost and suitable for industrial production of glycerophosphocholine.
METHOD OF PREPARING CHOLINE ALFOSCERATE AND PHARMACEUTICAL COMPOSITION COMPRISING THE SAME
-
Paragraph 0113-0141, (2020/10/27)
The present invention relates to a method for preparing choline alfoscerate. According to the method provided by the present invention, choline alfoscerate of high purity can be obtained in high yield, and a choline alfoscerate pharmaceutical formulation of excellent quality can be prepared using the same. In addition, the present invention is capable of obtaining high-purity choline alfoscerate in a high yield, as well as having excellent process efficiency as a preparation process is simple and short.COPYRIGHT KIPO 2021
Synthesis method for compound choline alfoscerate for promoting brain functions
-
Paragraph 0025, (2019/11/21)
The invention discloses a synthesis method for a compound choline alfoscerate for promoting brain functions. The synthesis method comprises the following steps: (1) enabling calcium phosphorylcholinechloride to react with oxalic acid to generate precipitate of phosphorylcholine chloride and oxalic acid; (2) enabling the phosphorylcholine chloride prepared in the step (1) to react with an alkali in a solvent so as to obtain a salt of the phosphorylcholine chloride; and (3) enabling the salt of the phosphorylcholine chloride to react with propylene glycol, so as to obtain choline alfoscerate. Raw materials of the synthesis method for the compound choline alfoscerate for promoting brain functions are low in price, low in cost and easy to obtain, the synthesis method is short in synthesis path and high in yield, the obtained product is high in chemical purity, all reactions need no special production equipment, the obtained intermediate and final products need no column chromatography orcrystallization purification, the production cost can be lowered, industrial amplified production can be facilitated, a high-purity product can be provided for the market, and thus high economic benefits can be met.
Preparation method of L-alpha-glycerophosphorylcholine
-
Paragraph 0035; 0037; 0046; 0048; 0049; 0056-0058, (2019/01/08)
The invention relates to the field of L-alpha-glycerophosphorylcholine and particularly relates to a preparation method of high-purity L-alpha-glycerophosphorylcholine. The preparation method uses glycidol as a raw material to prepare L-alpha-glycerophosphorylcholine. The preparation method comprises: preparing 2-chloro-1, 3, 2-dioxaphospholane from phosphorus trichloride and ethylene glycol, carrying out oxidization, adding trimethylamine and dioxaphospholane for a reaction, adding an acid into the reaction system, carrying out ring opening oxidization to obtain L-alpha-glycerophosphorylcholine chloride, and adding resin into the L-alpha-glycerophosphorylcholine chloride to remove chloride ions so that a finished product is obtained. The preparation method has the advantages of mild reaction conditions, short reaction time, easy availability of raw materials, simple post-treatment and high product purity.
28319-77-9 Process route
-
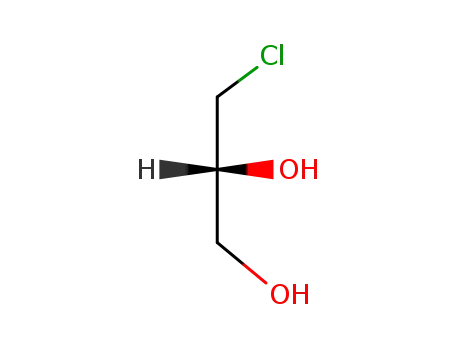
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

-
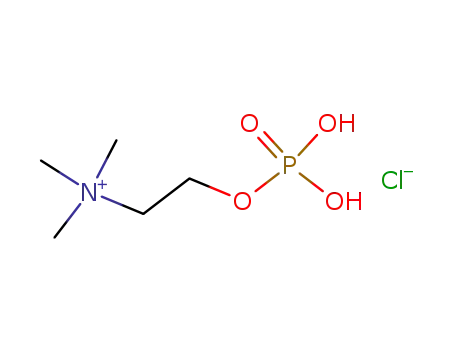
-
107-73-3
phosphorylcholine chloride

-
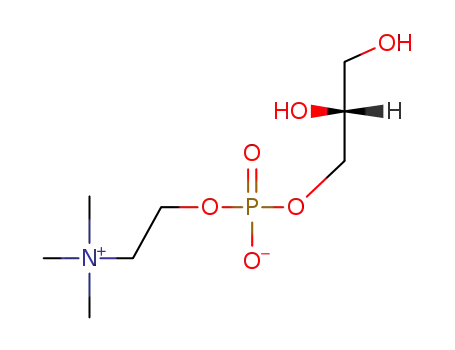
-
28319-77-9,563-24-6
L-glycero-3-phosphorylcholine
| Conditions | Yield |
|---|---|
|
phosphorylcholine chloride;
With
potassium hydroxide;
In
methanol;
for 1h;
(2R)-3-chloro-1,2-propanediol;
In
methanol;
at 65 ℃;
for 16h;
Temperature;
|
99% |
|
phosphorylcholine chloride;
With
potassium hydroxide;
In
ethanol;
for 0.5h;
(2R)-3-chloro-1,2-propanediol;
In
ethanol;
at 75 - 85 ℃;
for 6h;
|
79.6% |
-
-
C8H21NO6P(1+)*Cl(1-)

-

-
28319-77-9,563-24-6
L-glycero-3-phosphorylcholine
| Conditions | Yield |
|---|---|
|
With
717 type ion exchange resin;
In
ethanol; water;
at 0 ℃;
for 4h;
|
77% |
28319-77-9 Upstream products
-
10417-94-4
all cis-5,8,11,14,17-eicosapentaenoic acid
-
63-89-8
L-dipalmitoylphosphatidylcholine
-
54672-38-7
(R)-1,2-diacetoxy glycerylphosphoryl choline
-
1417365-52-6
siladenoserinol A
28319-77-9 Downstream products
-
18194-24-6
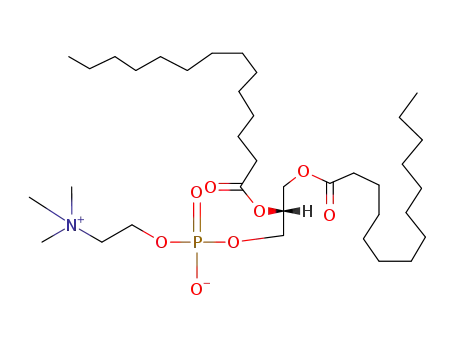
dimyristoylphosphatidylcholine
-
4235-95-4
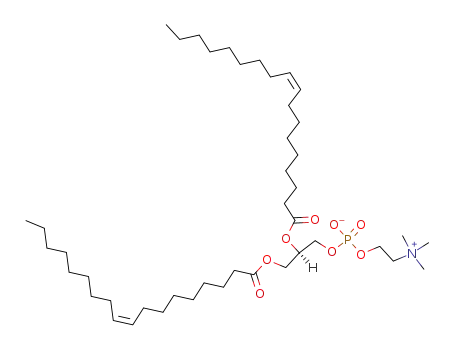
dioleoylphosphatidylcholine
-
4235-95-4
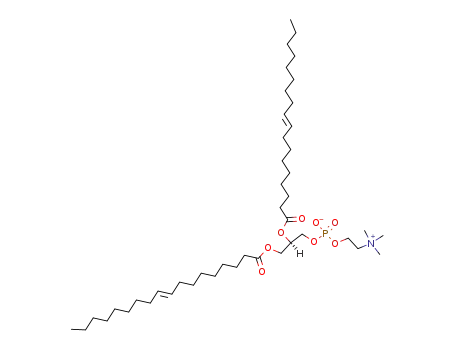
1,2-dioleoyl-sn-glycero-3-phosphocholine
-
3436-44-0
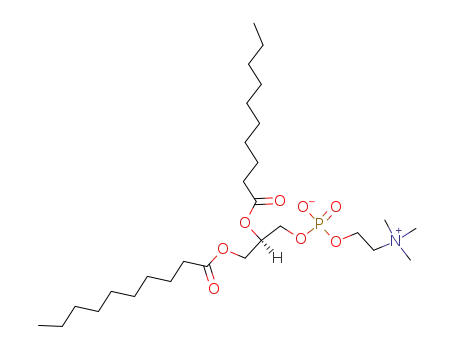
1,2-didecanoyl-sn-glycero-3-phosphocholine








