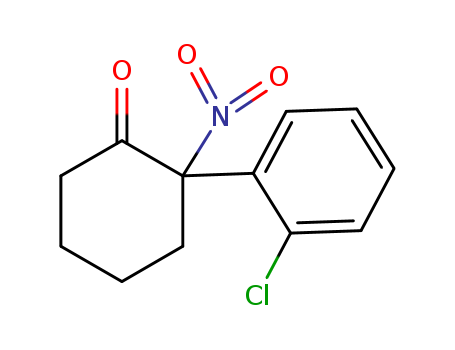
2-(2-Chlorophenyl)-2-nitrocyclohexanone 2079878-75-2
- CasNo:2079878-75-2
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
2079878-75-2 Properties
- Molecular Formula:C12H12ClNO3
- Molecular Weight:253.685
2079878-75-2 Relevant articles
NMDA receptor antagonist and use thereof
-
Paragraph 0367; 0369; 0372-0373, (2021/08/11)
The present invention relates to an NMDA receptor antagonist and use thereof. The NMDA receptor antagonist is a compound as shown in the formula I, and pharmaceutically acceptable salts, enantiomers, diastereoisomers, tautomers, solvates, isotope substitutes, polymorphic substances, prodrugs or metabolites thereof, and in the formula, ring A, ring B and R2 are as described in the specification. The invention also provides pharmaceutical compositions containing the compounds, and applications of the compounds in preparation of drugs for treating or preventing NMDA receptor mediated diseases.
Transition-metal-free α-arylation of nitroketones with diaryliodonium salts for the synthesis of tertiary α-aryl, α-nitro ketones
An, Yang,Zhang, Xiao-Ming,Li, Ze-Yu,Xiong, Wen-Hui,Yu, Run-Dong,Zhang, Fu-Min
supporting information, p. 119 - 122 (2019/01/03)
Transition-metal-free α-arylation of α-nitroketones with diaryliodonium salts has been realized for the first time. As an application of this methodology, a concise synthesis of the clinical drug tiletamine was also achieved via a three-step procedure from 2-nitrocyclohexanone without the isolation of intermediates.
Copper-Assisted Direct Nitration of Cyclic Ketones with Ceric Ammonium Nitrate for the Synthesis of Tertiary α-Nitro-α-substituted Scaffolds
Zhang, Zhi-Qiang,Chen, Tao,Zhang, Fu-Min
supporting information, p. 1124 - 1127 (2017/03/15)
An efficient and direct Cu-assisted nitrating approach to create synthetically valuable and challenging tertiary α-nitro-α-substituted moieties has been developed using ceric ammonium nitrate as a nitrating reagent, oxidant, and Lewis acid. Notably, the commonly used clinical drug ketamine was smoothly synthesized in four steps.
Intermediate compound for synthesizing ketamine and method for synthesizing ketamine
-
Paragraph 0043; 0048; 0049, (2017/08/28)
The invention discloses an intermediate compound for synthesizing ketamine, having a chemical structural formula shown in the specification. The intermediate compound is prepared by: (i) reacting o-chlorobromobenzene and lithium alkylide to obtain lithium o-chlorophenyl; (ii) reacting the lithium o-chlorophenyl and cyclohexene oxide under the action of Lewis acid. The intermediate compound may be oxidized, nitrified, nitro-reduced and then amino-methylated to produce ketamine. Compared with existing synthetic methods of ketamine, the method of the invention has simple route, low cost and good operation convenience.
2079878-75-2 Process route
-
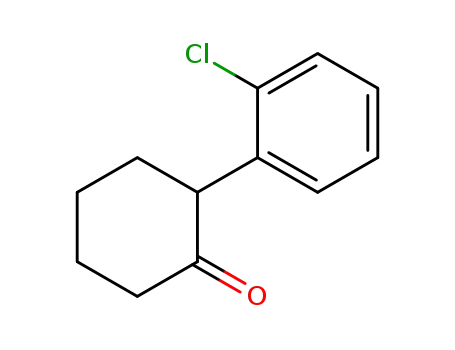
-
91393-49-6
2-amino-2-(2-chlorophenyl)cyclohexan-1-one

-
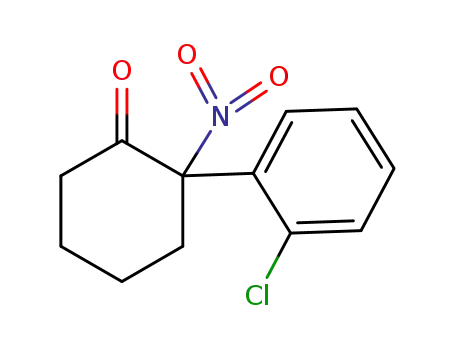
-
2079878-75-2
2-(2-chlorophenyl)-2-nitrocyclohexan-1-one
| Conditions | Yield |
|---|---|
|
With
ammonium cerium (IV) nitrate; copper diacetate;
In
1,2-dichloro-ethane;
at 80 ℃;
for 12h;
Inert atmosphere;
Sealed tube;
|
51% |
|
With
ammonium cerium (IV) nitrate; copper diacetate;
In
1,2-dichloro-ethane;
at 100 ℃;
for 12h;
Inert atmosphere;
Sealed tube;
|
51% |
|
With
ammonium cerium (IV) nitrate; copper diacetate;
In
1,2-dichloro-ethane;
at 80 ℃;
for 12h;
|
-
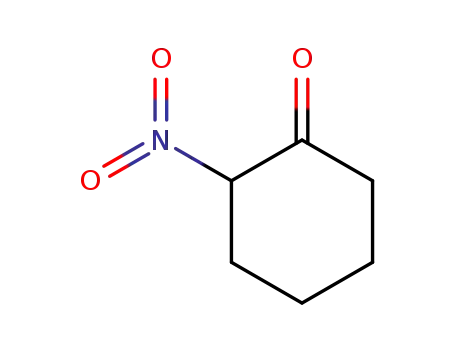
-
4883-67-4
2-nitrocyclohexanone

-
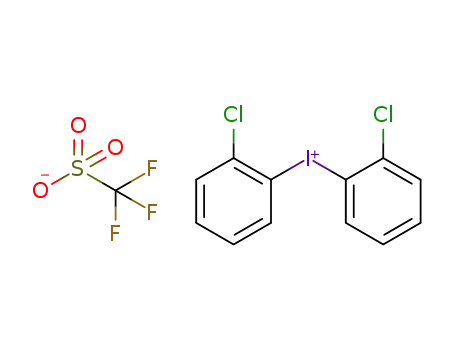
-
trifluoromethanesulfonic acid di(o-chlorophenyl)iodonium salt

-

-
2079878-75-2
2-(2-chlorophenyl)-2-nitrocyclohexan-1-one
| Conditions | Yield |
|---|---|
|
2-nitrocyclohexanone;
With
potassium carbonate;
In
1,2-dichloro-ethane;
at 20 ℃;
for 0.166667h;
Inert atmosphere;
trifluoromethanesulfonic acid di(o-chlorophenyl)iodonium salt;
In
1,2-dichloro-ethane;
at 80 ℃;
Inert atmosphere;
|
41% |
2079878-75-2 Upstream products
-
91393-49-6

2-amino-2-(2-chlorophenyl)cyclohexan-1-one
-
694-80-4
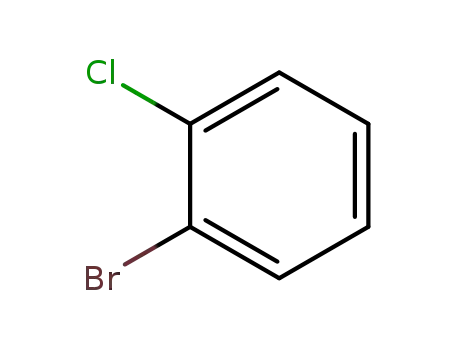
2-bromo-1-chlorobenzene
-
286-20-4
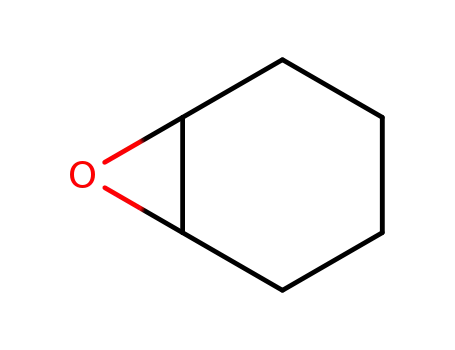
cyclohexane-1,2-epoxide
-
4883-67-4

2-nitrocyclohexanone
2079878-75-2 Downstream products
-
35211-10-0
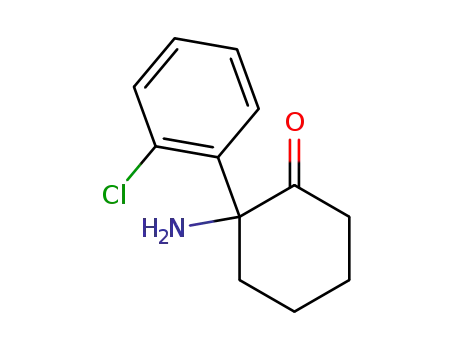
norketamine
-
6740-88-1
ketamine








