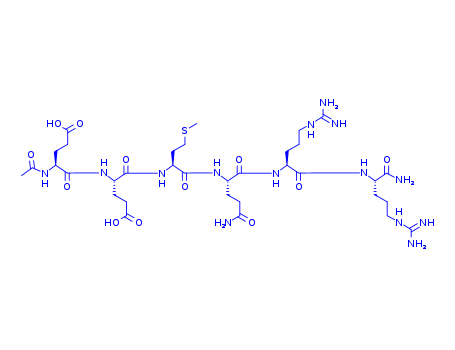
Acetyl Hexapeptide-8 Cas No.616204-22-9
- CasNo:616204-22-9
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
616204-22-9 Properties
- Molecular Formula:C34H60N14O12S
- Molecular Weight:889.003
- Appearance/Colour:white powder
- Refractive Index:1.66
- PKA:4.43±0.10(Predicted)
- PSA:484.48000
- Density:1.54 g/cm3
- LogP:0.80300
616204-22-9 Usage
Description
Argireline is made up of chains of amino acids. Such chains can affect how our cells work, e.g. relaxing our face muscles. It is derived from the peptide Acetyl hexapeptide-3, a substrate of Botulinum toxin, or more commonly known as Botox.
Application
Botox is a common, anti-wrinkle treatment which is applied using a needle. You may achieve the same anti-aging results with products which contain Argireline. Argireline can help prevent the formation of wrinkles by inhibiting muscle movement in the face. Because of this, Argireline cream is sometimes known as “Botox in a jar.”
Uses
Argireline is an anti-wrinkle peptide. Synthetically produced and considered to be highly effective, clinical studies indicate it can reduce the depth of existing wrinkles. May be incorporated into products marketed as having a topical-BoToX. or “wrinkle-erasing” effect.
benefits
Acetyl Hexapeptide-8 is effectivly reduce various kind of facial wrinkles;Replenish the collagen and polysaccharide, repairthe skin.Winkey patented productin China.It is recommended dosage is 3~10%.
Clinical claims and research
Argireline is a synthetic oligopeptide that emulates the sequence of SNAP-25, a type of SNARE. It has significant skin permeation, interferes with the SNARE ternary complex, and has demonstrated interference with catecholamine release from chromaffin cells. Topical use of 10% argireline resulted in a reduced depth and roughness of periorbital wrinkles in 10 healthy women. One study on 14 volunteers showed that 5% argireline reduced periorbital wrinkles by 32% in 28 days. Most recently, argireline’s efficacy has been measured in 60 Chinese women from a randomized control trial. Its total efficacy was 48.9% compared to 0% in the placebo group, and resulted in significantly decreased wrinkle roughness.
InChI:InChI=1/C34H60N14O12S/c1-17(49)43-20(8-11-25(51)52)29(57)47-22(9-12-26(53)54)31(59)48-23(13-16-61-2)32(60)46-21(7-10-24(35)50)30(58)45-19(6-4-15-42-34(39)40)28(56)44-18(27(36)55)5-3-14-41-33(37)38/h18-23H,3-16H2,1-2H3,(H2,35,50)(H2,36,55)(H,43,49)(H,44,56)(H,45,58)(H,46,60)(H,47,57)(H,48,59)(H,51,52)(H,53,54)(H4,37,38,41)(H4,39,40,42)/t18-,19-,20-,21-,22-,23-/m0/s1
616204-22-9 Relevant articles
Sustainable Peptide Synthesis Enabled by a Transient Protecting Group
Avrutina, Olga,Knauer, Sascha,Koch, Niklas,Kolmar, Harald,Meusinger, Reinhard,Uth, Christina
supporting information, p. 12984 - 12990 (2020/06/01)
The growing interest in synthetic peptides has prompted the development of viable methods for their sustainable production. Currently, large amounts of toxic solvents are required for peptide assembly from protected building blocks, and switching to water as a reaction medium remains a major hurdle in peptide chemistry. We report an aqueous solid-phase peptide synthesis strategy that is based on a water-compatible 2,7-disulfo-9-fluorenylmethoxycarbonyl (Smoc) protecting group. This approach enables peptide assembly under aqueous conditions, real-time monitoring of building block coupling, and efficient postsynthetic purification. The procedure for the synthesis of all natural and several non-natural Smoc-protected amino acids is described, as well as the assembly of 22 peptide sequences and the fundamental issues of SPPS, including the protecting group strategy, coupling and cleavage efficiency, stability under aqueous conditions, and crucial side reactions.
COMPOUNDS USEFUL FOR THE TREATMENT AND/OR CARE OF THE SKIN, HAIR, NAILS AND/OR MUCOUS MEMBRANES
-
Paragraph 0167; 0178-0182; 0185-0188, (2018/06/21)
A compound of formula (I) R1-Wm-Xn-AA1-AA2-AA3-AA4-AA5-AA6-(AA7)a-(AA8)b-Yp-Zq-R2 effective in maintaining and/or improving the physical and immunological barrier functions of the skin and useful in the treatment and/or care of the skin, hair, nails and/or mucous membranes.
616204-22-9 Process route
-
-
C20H18N2O11S2(2-)*2Na(1+)

-
-
C24H25NO12S2(2-)*2Na(1+)

-
-
C20H19NO10S3(2-)*2Na(1+)

-
-
C21H22N4O10S2(2-)*2Na(1+)

-
-
616204-22-9
Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2
| Conditions | Yield |
|---|---|
|
C21H22N4O10S2(2-)*2Na(1+);
With
sodium hydrogencarbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; ethyl cyanoglyoxylate-2-oxime;
In
water;
at 20 ℃;
for 0.75h;
With
sodium hydroxide;
In
water;
for 0.0833333h;
C20H18N2O11S2(2-)*2Na(1+); C24H25NO12S2(2-)*2Na(1+); C20H19NO10S3(2-)*2Na(1+); C21H22N4O10S2(2-)*2Na(1+); acetyl source;
Further stages;
|
42.16% |
-
-
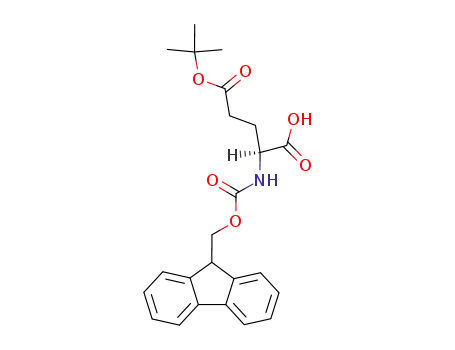
71989-18-9
Fmoc-Glu(OtBu)-OH

-
-
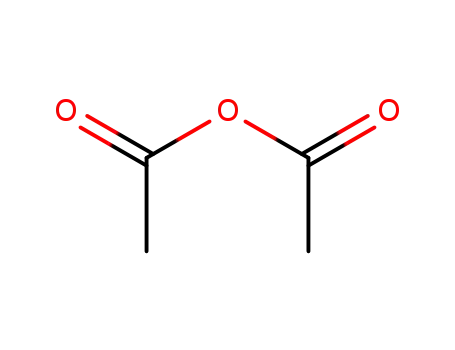
108-24-7
acetic anhydride

-
![N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methionine](/upload/2023/2/0da8a26f-85d0-4382-b3d9-7bdea4e8e468.png)
-
71989-28-1
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methionine

-
-
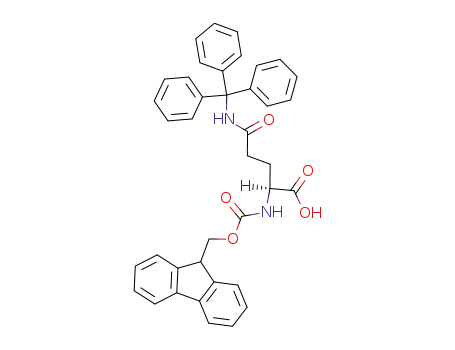
132327-80-1,200623-62-7
Fmoc-L-Gln(Trt)-OH

-
-
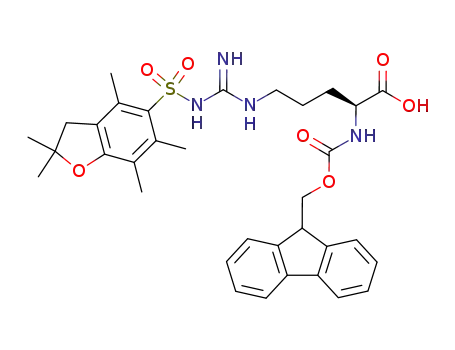
187618-60-6
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine

-
-
616204-22-9
Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2
| Conditions | Yield |
|---|---|
|
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine;
With
benzotriazol-1-ol; diisopropyl-carbodiimide;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 1h;
With
piperidine;
In
N,N-dimethyl-formamide;
at 25 ℃;
Fmoc-Glu(OtBu)-OH; acetic anhydride; N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methionine; Fmoc-L-Gln(Trt)-OH; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine;
Further stages;
|
616204-22-9 Upstream products
-
71989-18-9

Fmoc-Glu(OtBu)-OH
-
108-24-7

acetic anhydride
-
71989-28-1
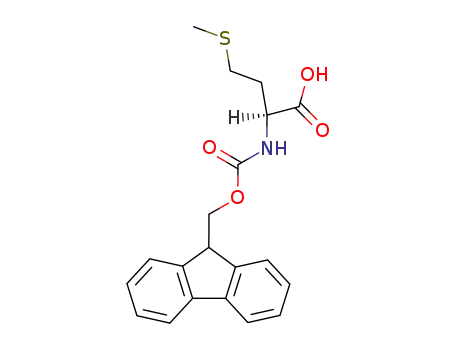
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methionine
-
132327-80-1

Fmoc-L-Gln(Trt)-OH








