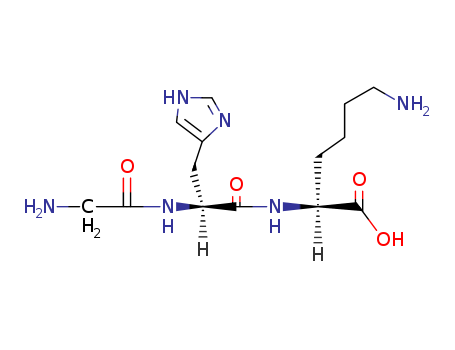
Copper Tripeptide-1 Cas No.49557-75-7
- CasNo:49557-75-7
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
49557-75-7 Properties
- Molecular Formula:C14H24N6O4
- Molecular Weight:340.382
- Appearance/Colour:White powder
- Vapor Pressure:2.86E-29mmHg at 25°C
- Refractive Index:1.582
- Boiling Point:831 °C at 760 mmHg
- PKA:3.11±0.10(Predicted)
- Flash Point:456.4 °C
- PSA:176.22000
- Density:1.324 g/cm3
- LogP:0.27650
49557-75-7 Usage
Description
Copper peptide GHK-Cu is a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine. The tripeptide has strong affinity for copper(II) and was first isolated from human plasma. It can be found also in saliva and urine.
Uses
Copper Peptide(GHK-Cu) can be used as skin antiaging agents.
Uses
anti-wrikle
Uses
Glycyl-L-histidyl-L-lysine is a liver cell growth factor and an asynthetic hepatotrophic agent that stimulates hepatic erythropoietic factor production.
Definition
ChEBI: A tripeptide composed of glycine, L-histidine and L-lysine residues joined in sequence.
Pharmaceutical Applications
Glycyl-l-histidyl-l-lysine (GHK) is a tripeptide known for its high binding affinity to Cu2+ and its complex role in wound healing. The GHK–Cu(II) complex was isolated from human plasma in the 1970s and it was shown to be an activator for wound healing. GHK–Cu(II) has two main functions: as an anti-inflammatory agent to protect the tissue from oxidative damage after the injury, and as an activator for wound healing itself as it activates the tissue remodelling. The structure of GHK is very similar to that of common drugs used to treat ulcers. After the initial stages of wound healing are activated, such as blood coagulation and neutrophil invasion, a second stage of wound healing begins, which includes the population of GHK at the wound, which has a high affinity to Cu2+. Mast cells, which are located in the skin, secrete GHK, which accumulates Cu2+ and forms the copper complex GHK–Cu(II) and therefore increases the metal–tripeptide concentration at the wound. First, GHK–Cu(II) has an anti-inflammatory effect by protecting the tissue from oxidative damage and by suppressing local inflammatory signals (i.e. cytokine interleukin-1 (IL-1)). Second, GHK–Cu(II) is released into the blood stream and encourages the production of wound macrophages that support the wound repair by removing the damaged tissue and secreting a family of several growth factor proteins. GHK–Cu(II) also hinders fibroblast production of TGF-β-1 and therefore suppresses the scar development. The GHK–Cu(II) complex also stimulates the growth of blood vessels, neurons and elastin, and, in general, supports most processes of wound healing.
InChI:InChI=1/C14H24N6O4/c15-4-2-1-3-10(14(23)24)20-13(22)11(19-12(21)6-16)5-9-7-17-8-18-9/h7-8,10-11H,1-6,15-16H2,(H,17,18)(H,19,21)(H,20,22)(H,23,24)
49557-75-7 Relevant articles
Solid-phase synthesis, characterization and DNA binding properties of the first chloro(polypyridyl)ruthenium conjugated peptide complex
Karidi, Konstantina,Garoufis, Achilleas,Hadjiliadis, Nick,Reedijk, Jan
, p. 728 - 734 (2005)
A general method for the synthesis of chloro(polypyridyl)ruthenium conjugated peptide complexes via a solid-phase strategy is described. The method is applied to synthesize two positional isomers of the complex [Ru(terpy)(4-CO2H-4'-Mebpy-Gly-L-His-L-LysCONH2)Cl] (PF6). Even though the separation of the isomers was only partially achieved chromatographically, the isomers were unambiguously assigned by NMR spectroscopy. The interactions of the complex [Ru(terpy)(4-CO 2H-4'-Mebpy-Gly-L-His-L-LysCONH2)Cl](PF6) with CT-DNA and plasmid DNA, have been studied with various spectroscopic techniques, showing that (i) the complexes coordinatively bind to DNA preferring the bases guanine and cytosine over the bases thymine and adenine after hydrolysis of the coordinated chloride, (ii) electrostatic interactions between the complex cation and the polyanionic DNA chain assist this binding (iii) only in the case of one isomer the peptide does interact further with DNA as evidenced from 31P NMR spectroscopy, (iv) DNA unwinding occurs in all cases with high binding ratio (Ru/base) values (r > 0.3). The Royal Society of Chemistry 2005.
Solid phase method for synthesis peptide-spacer-lipid conjugates, conjugates synthesized thereby and targeted liposomes containing the same
-
Page 12; 13, (2010/02/03)
A solid phase synthesis method for preparing peptide-spacer-lipid conjugates, the peptide-spacer-lipid conjugates synthesized by the method, and liposomes containing the peptide-spacer-lipid conjugates. The present invention provides a convenient solid phase synthesis method for preparing peptide-spacer-lipid conjugates and provides various linkage groups (such as amide group) for conjugating peptide, spacer and lipid, wherein the spacer may comprise PEG. Several advantages can be achieved, such as the synthetic procedure can be simplified, the synthesis process can be set to automation, the purification is easier in each reaction step, and the product losses can be reduced to minimal during synthesis. The present synthesis method is suitable for preparing a wide range of peptide-spacer-lipid conjugates, provides a peptide-spacer-lipid conjugate prepared by the solid phase synthesis method of the present invention, which can be incorporated into a liposome as the targeting moiety for liposomal drug delivery to specific cells, and provides a targeting liposome containing the present peptide-spacer-lipid conjugate.
Solid phase method for synthesis peptide-spacer-lipid conjugates, conjugates synthesized thereby and targeted liposomes containing the same
-
Page 13, (2010/02/03)
A solid phase synthesis method for preparing peptide-spacer-lipid conjugates, the peptide-spacer-lipid conjugates synthesized by the method, and liposomes containing the peptide-spacer-lipid conjugates. The present invention provides a convenient solid phase synthesis method for preparing peptide-spacer-lipid conjugates and provides various linkage groups (such as amide group) for conjugating peptide, spacer and lipid, wherein the spacer may comprise PEG. Several advantages can be achieved, such as the synthetic procedure can be simplified, the synthesis process can be set to automation, the purification is easier in each reaction step, and the product losses can be reduced to minimal during synthesis. The present synthesis method is suitable for preparing a wide range of peptide-spacer-lipid conjugates, provides a peptide-spacer-lipid conjugate prepared by the solid phase synthesis method of the present invention, which can be incorporated into a liposome as the targeting moiety for liposomal drug delivery to specific cells, and provides a targeting liposome containing the present peptide-spacer-lipid conjugate.
Bursopoietin
-
, (2008/06/13)
A synthetic peptide which possesses the ability to specifically induce the differentiation of precursor B cells into mature B-cells capable of producing antibody.
49557-75-7 Process route
-
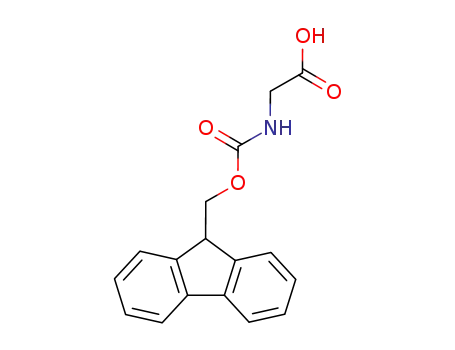
-
29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine

-
-
71989-26-9
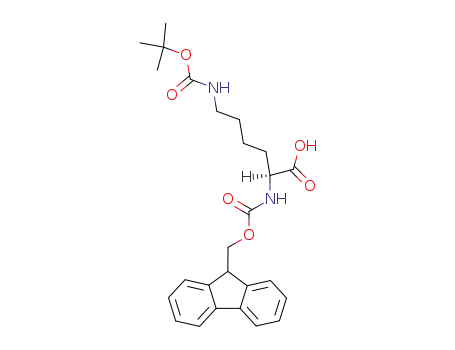
Fmoc-Lys(tert-butoxycarbonyl)

-
-
109425-51-6
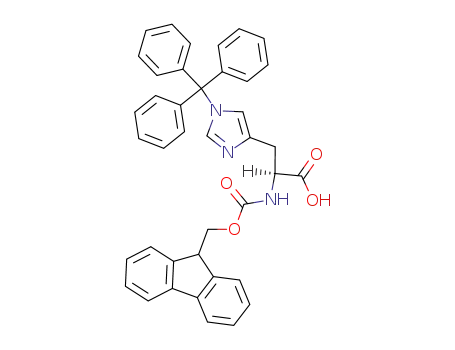
Fmoc-His(Trt)-OH

-
-
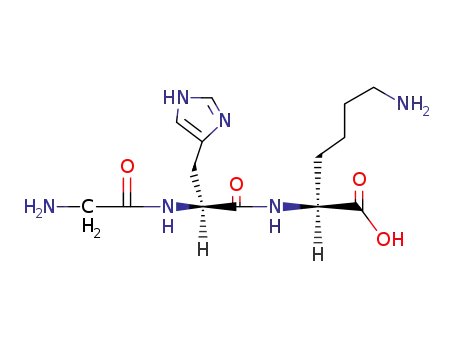
49557-75-7
glycyl-L-histidyl-L-lysine
| Conditions | Yield |
|---|---|
|
Multistep reaction;
|
40% |

-
-
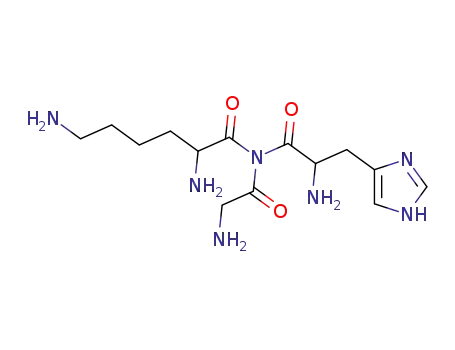
glycyl-L-histidyl-L-lysineamide

-

-
49557-75-7
glycyl-L-histidyl-L-lysine
| Conditions | Yield |
|---|---|
|
|
49557-75-7 Upstream products
-
29022-11-5

N-(fluoren-9-ylmethoxycarbonyl)glycine
-
71989-26-9

Fmoc-Lys(tert-butoxycarbonyl)
-
109425-51-6

Fmoc-His(Trt)-OH