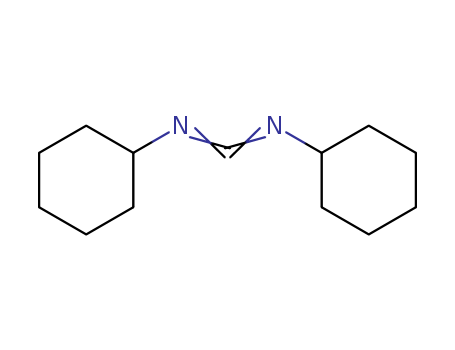
N'n-dicyclohexyl carbodiimide 538-75-0
- CasNo:538-75-0
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
538-75-0 Properties
- Molecular Formula:C13H22N2
- Molecular Weight:206.331
- Appearance/Colour:colorless solid
- Vapor Pressure:1.044-1.15Pa at 20-25℃
- Melting Point:34-35 °C(lit.)
- Refractive Index:n20/D 1.48
- Boiling Point:277 °C at 760 mmHg
- Flash Point:113.1 °C
- PSA:24.72000
- Density:1.06 g/cm3
- LogP:3.82570
538-75-0 Usage
Description
Dicydohexyl carbodiimide is used in peptide chemistry as a coupling reagent. It is both an irritant and a sensitizer, and caused contact dermatitis in pharmacists and chemists.
Chemical Properties
Colorless solid
Chemical Properties
N,N0 -Dicyclohexylcarbodiimide (DCC) is a white crystalline solid. Odor is sweet and heavy.
Uses
In the synthesis of peptides.
Uses
This product is mainly used in amikacin, glutathione dehydrants, as well as in synthesis of acid anhydride, aldehyde, ketone, isocyanate; when it is used as dehydrating condensing agent, it reacts to dicyclohexylurea through short-time reaction under normal temperature. This product can also be used in synthesis of peptide and nucleic acid. It is easy to use this product to react with compound of free carboxy and amino-group into peptide. This product is widely used in medical, health, make-up and biological products, and other synthetic fields.
Uses
N,N'-Dicyclohexylcarbodiimide is a carbodiimide used to couple amino acids during peptide synthesis. N,N'-Dicyclohexylcarbodiimide is used as a dehydrating agent for the preparation of amides, ketones , nitriles as well as in the inversion and esterification of secondary alcohols.
Uses
dicyclohexylcarbodiimide is used as a dehydrating agent at room temperature after a short reaction time, after the reaction product is dicyclohexylurea. the product is very small solubility in an organic solvent, so that easy separation of the reaction product.
Definition
ChEBI: A carbodiimide compound having a cyclohexyl substituent on both nitrogen atoms.
Preparation
A stirred mixture of N,N′-dicyclohexylurea (19.7 g), phosphorus pentoxide (100 g), sand (175 g), and pyridine (700 mL) was refluxed for 2.25 h. Stirring was no longer possible after about 30 min. The mixture was filtered and the residue was extracted with pyridine (100 mL). Pyridine was removed from the combined solutions on a flash evaporator, and the residual oil was extracted with boiling petroleum ether (bp 60–80 C°) (2 × 100 mL), and then with diethyl ether (100 mL). The combined extracts were washed with iced water (3×80 mL), dried over calcium chloride, and filtered. The solvents were removed from the filtrate under reduced pressure to give 17.4 g of an oil, which on distillation yielded 13.7 g (76%) of a clear liquid; bp 143 C° (3.5 mmHg), which solidified in the receiver; mp 34–35 C°. Another method for producing DCC from dicyclohexylurea is a two-step process using phosphoryl chloride in dichloromethane at 40 C° for 4 h under non-basic conditions followed by removal of acidic components with aq. sodium hydroxide. This method, which gives an 89% yield of DCC, has been presented in a patent application.
Preparation
Palladium acetate (22 mg, 0.1 mmol), iodine (50 mg, 0.2 mmol), and anhydrous sodium carbonate (320 mg, 3.0 mmol) were placed in a pressure vessel. Cyclohexylamine (0.11 mL, 1.0 mmol) and cyclohexyl isocyanide (0.1 mL, 0.8 mmol) were dissolved in acetonitrile (10 mL) and this solution was added to the reaction vessel, which was then pressurized with oxygen (40 psi) and heated to 100 C° for 3 h. The initially deep-red reaction mixture turned yellow-orange; no Pd black precipitation was observed. There was no obvious reaction rate dependence on oxygen pressure. The mixture was cooled to ambient temperature, depressurized, filtered, and analyzed by GC. DCC was isolated by evaporating the solvent and residual amine, followed by vacuum distillation. Palladium(II) complexes with a carbodiimide ligand, in which a nitrogen of the linear NbCbN moiety is bonded to the metal center, and bis(carbodiimido)palladium(II) complexes, both derived from isocyanides, have been described.
General Description
White crystalline solid with a heavy sweet odor.
Air & Water Reactions
Sensitive to moisture.
Reactivity Profile
Dicyclohexylcarbodiimide is an imide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Dicyclohexylcarbodiimide is incompatible with acids and oxidizing agents. Dicyclohexylcarbodiimide reacts with water.
Hazard
A poison by skin contact. Moderately toxic by ingestion and inhalation.
Fire Hazard
Dicyclohexylcarbodiimide is probably combustible.
Flammability and Explosibility
Notclassified
Contact allergens
Used in peptide chemistry as a coupling reagent. It is both an irritant and a sensitizer and has caused contact dermatitis in pharmacists and chemists.
Potential Exposure
Laboratory reagent
Shipping
UN2928 Toxic solids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8- Corrosive material, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required
Purification Methods
It is sampled as a liquid after melting in warm H2O. It is sensitive to air, and it is a potent skin irritant. It can be distilled in a vacuum and is best stored in a tightly stoppered flask in a freezer. It is very soluble in CH2Cl2 and pyridine where the reaction product with H2O, after condensation, is dicyclohexyl urea which is insoluble and can be filtered off. Alternatively dissolve it in CH2Cl2, add powdered anhydrous MgSO4, shake for 4hours, filter, evaporate and distil at 0.6mm pressure and oil bath temperature of 145o. [Bodansky et al. Biochemical Preparations 10, 122 1963, Schmidt & Seefelder Justus Liebigs Ann Chem 571 83 1951, Schmidt et al. Justus Liebigs Ann Chem 612 11 1958, Beilstein 12 IV 72.]
Incompatibilities
Dust may for explosive mixture with air. Reacts with steam and water. N,N0 - Dicyclohexylcarbodiimide is an amine/imide: contact with strong oxidizers may cause fire and explosions. Incompatible with acids, strong bases, strong reducing agents (may form flammable gasses); azo and diazo compounds (may form toxic gases); chlorinated hydrocarbons; nitro compounds. Contact with mixture of acetic acid 1 dinitrogen trioxide may cause explosion. The combustion of amide compounds generate nitrogen oxides (NOx). In the presence of moisture, may attack metals and plastics.
Waste Disposal
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste facility. Although not a listed RCRA hazardous waste, this material may exhibit one or more characteristics of a hazardous waste and require appropriate analysis to determine specific disposal requirements. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirements
538-75-0 Relevant articles
MIGRATION REVERSIBLE DE PHOSPHORYLE S-P N-P CONTROLEE PAR TRANSFERT DE PROTON, MODELE D'ACTIVATION PAR PHOSPHORYLATION
Blonski, C.,Gasc, M. B.,Klaebe, A.,Perie, J. J.
, p. 2773 - 2776 (1982)
This work describes the reversible C-addition of a sterically hindered-P thiophosphoric ester to a carbodiimide, as a model of activated biotin.The formed intermediate, is found to be in equilibrium with the corresponding N-phosphorylated thiourea.The entire system is controlled by proton transfer and reversible.
Stimuli-Responsive Frustrated Lewis-Pair-Type Reactivity of a Tungsten Iminoazaphosphiridine Complex
Villalba Franco, José Manuel,Schnakenburg, Gregor,Sasamori, Takahiro,Espinosa Ferao, Arturo,Streubel, Rainer
, p. 9650 - 9655 (2015)
Reactions of 3-imino-azaphosphiridine complexes 1 a,b with carbodiimides 2 a,b, isocyanates 3 a,b, and carbon dioxide are described. Whereas exchange of the carbodiimide unit occurs in the first case, an overall ring expansion takes place with phenyl isocyanate (3 a) and carbon dioxide to yield complexes 4 and 5 bearing novel 1,3,5-oxazaphospholane ligands; the isopropyl derivative 3 b did not react under these conditions. DFT calculations provide insight into the pathway of the reaction with carbon dioxide with model complex 1 c, revealing effects of initial non-covalent interactions with the substrate onto the ring bonding, thus triggering an initially masked frustrated Lewis-pair-type behavior.
Method for preparing N,N'-dicyclohexylcarbodiimide by using co-reactor
-
Paragraph 0043-0046, (2021/03/30)
The invention discloses a method for preparing N,N'-dicyclohexylcarbodiimide by using a co-reactor. The method comprises the steps of maintaining an aqueous solution of cyclohexylamine in a strong alkaline environment in the same reactor, adding carbon disulfide into the same reactor, and adding sodium hypochlorite for oxidation to obtain N,N'-dicyclohexylcarbodiimide. According to the method, thetechnological process is simplified through the co-reactor reaction, generation of highly toxic and harmful gas is avoided, the safety risk of production is reduced, and then the purpose of environment-friendly and safe production is achieved.
Method for producing N,N'-dicyclohexyl carbodiimide
-
Paragraph 0013; 0027-0045, (2021/05/08)
The invention discloses a preparation method of N,N'-dicyclohexyl carbodiimide. The method comprises the following steps: taking dicyclohexyl urea (DCU) and trichloromethyl carbonate (BTC) as raw materials, dissolving dicyclohexyl urea (DCU) in a solvent, uniformly stirring, cooling, adding trichloromethyl carbonate and a catalyst loaded on a molecular sieve, carrying out heat preservation reflux, cooling, adjusting the pH value to 8-9 by using alkali, filtering the reaction liquid, evaporating out the solvent, and carrying out reduced pressure distillation to obtain the product namely N,N'-dicyclohexyl carbodiimide. According to the present invention, the problems of strong odor, strong toxicity and heavy environmental pollution of the traditional process are fundamentally eliminated, and the method has characteristics of high safety coefficient, partial catalyst recycling, high catalysis efficiency, partial raw material recycling, less three wastes, high purity and high yield, and is suitable for industrial production.
Microwave-assisted method for synthesizing N,N'-dicyclohexyl carbodiimide
-
Paragraph 0032-0051, (2021/04/17)
The invention relates to the technical field of organic synthesis, in particular to a method for synthesizing N,N'-dicyclohexyl carbodiimide through a microwave-assisted method. The method comprises the following steps: dissolving N,N'-dicyclohexyl urea in a solvent under microwave radiation, adding an oxidant and a catalyst loaded on a molecular sieve, and carrying out heat preservation reflux; adding a phase transfer catalyst, adjusting the pH value, and carrying out alkaline hydrolysis reaction; filtering, separating liquid, evaporating out the solvent, and carrying out reduced pressure distillation to obtain N,N'-dicyclohexyl carbodiimide. The catalyst loaded on the molecular sieve is large in specific surface area and high in catalytic efficiency; a microwave-assisted method is adopted, and a phase transfer catalyst is added, so that the problems of difficult two-phase reaction and incomplete reaction in the traditional process are solved, the reaction conversion rate is greatly improved, the reaction time is shortened, and the product has the characteristics of high purity and yield.
Method for preparing dicyclohexylcarbodiimide by using vilsmeier reagent
-
Paragraph 0015; 0021-0029, (2020/12/30)
The invention discloses a method for preparing dicyclohexylcarbodiimide by using a vilsmeier reagent. The method comprises the following steps: preparing a vilsmeier reagent by using N, N-dimethylformamide (DMF) and thionyl chloride (SOCl2), reacting the vilsmeier reagent with dicyclohexylurea (DCU), and carrying out aftertreatment to obtain the dicyclohexylcarbodiimide. According to the method, the vilsmeier reagent is adopted, the reaction can be carried out under mild conditions, the process is safe and stable, and the method is suitable for industrial large-scale production.
538-75-0 Process route
-
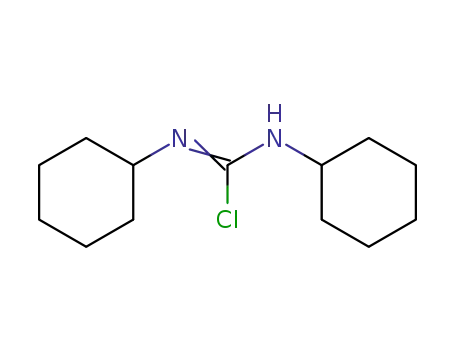
-
114316-64-2
N,N'-Dicyclohexyl-C-chlor-formamidinium

-
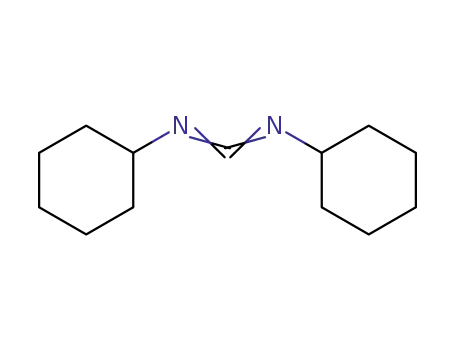
-
538-75-0
dicyclohexyl-carbodiimide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water;
|
92.4% |
-
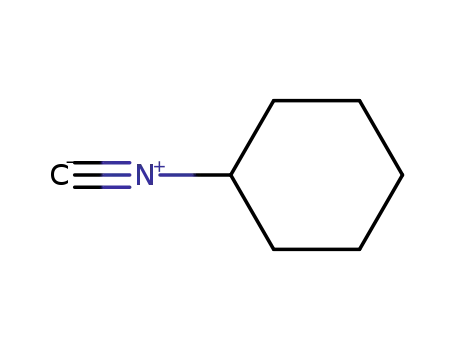
-
931-53-3
Cyclohexyl isocyanide

-
-
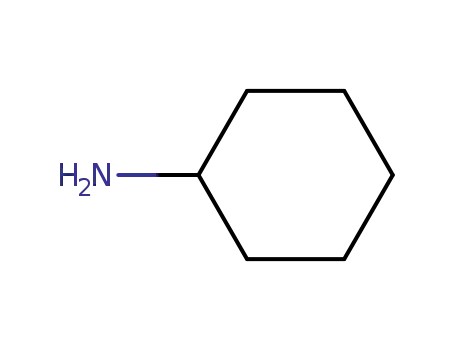
108-91-8,157973-60-9
cyclohexylamine

-
-
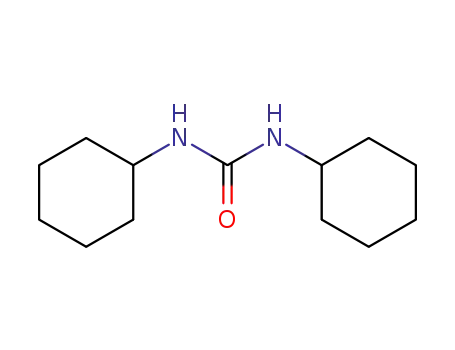
2387-23-7
1,3-Dicyclohexylurea

-

-
538-75-0
dicyclohexyl-carbodiimide
| Conditions | Yield |
|---|---|
|
With
oxygen; sodium carbonate;
palladium diacetate;
In
acetonitrile;
at 100 ℃;
for 3h;
under 2068.6 Torr;
|
35% 10 % Chromat. |
538-75-0 Upstream products
-
3173-53-3
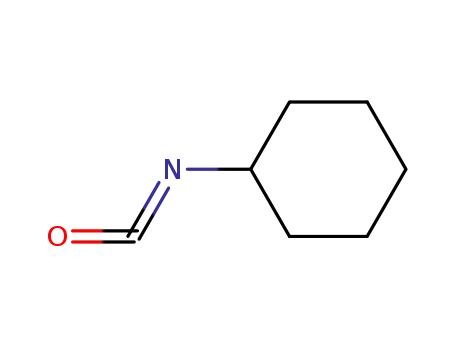
Cyclohexyl isocyanate
-
24901-29-9
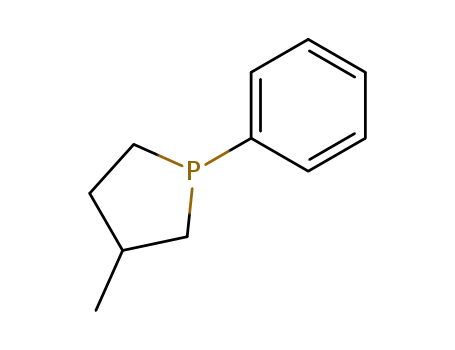
3-methyl-1-phenylphospholane
-
2387-23-7

1,3-Dicyclohexylurea
-
98-59-9
p-toluenesulfonyl chloride
538-75-0 Downstream products
-
25664-08-8
4-hydroxymethyl-2-oxo-2λ5-[1,3,2]dioxaphospholan-2-ol
-
93598-65-3
3-cyclohexyl-2-cyclohexylimino-6-methyl-2,3-dihydro-[1,3]oxazin-4-one
-
32449-99-3
indole-3-acetyl-N-aspartic acid
-
101865-05-8
N-(3,4-dimethoxyphenethyl)-2-(2-oxocyclohexyl)acetamide








