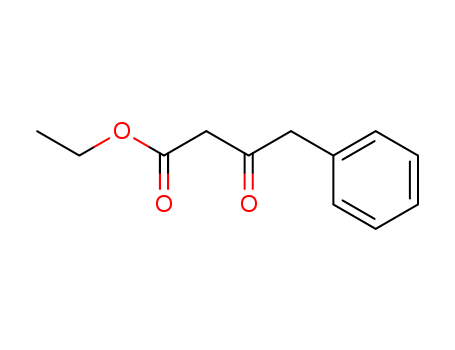
Ethyl3-oxo-4-phenylbutanoate 718-08-1
- CasNo:718-08-1
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
718-08-1 Properties
- Molecular Formula:C12H14O3
- Molecular Weight:206.241
- Vapor Pressure:0.00209mmHg at 25°C
- Refractive Index:1.054-1.059
- Boiling Point:290.3 °C at 760 mmHg
- PKA:10.49±0.46(Predicted)
- Flash Point:123.9 °C
- PSA:43.37000
- Density:1.091 g/cm3
- LogP:1.75140
718-08-1 Usage
Chemical Properties
Light-yellow Liquid.
Uses
Ethyl 3-oxo-4-phenylbutanoate is a useful synthetic intermediate. It is used to prepare pyrazolone derivatives as antiprion compounds. It is also used to prepare pyrrolinylaminopyrimidine analogs as inhibitors of AP-1 and NF-κB mediated gene expression.
Synthesis Reference(s)
Journal of Medicinal Chemistry, 44, p. 78, 2001 DOI: 10.1021/jm001034kChemical and Pharmaceutical Bulletin, 30, p. 2440, 1982 DOI: 10.1248/cpb.30.2440
Synthesis
The synthesis of Ethyl 3-oxo-4-phenylbutanoate is as follows:Monoethyl monopotassium malonate (12.9 g, 2.3 equivalents) was mixed with tetrahydrofuran (200 ml), and the mixture was cooled to 5°C. Triethylamine (8.2 g, 2.5 equivalents) and magnesium chloride (8.62 g, 2.8 equivalents) were added, and the mixture was stirred at 5 to 20°C for 3 hours. The reaction mixture was cooled to 5°C. Phenacyl chloride (5 g, 32 mmol, 1 equivalent) was gradually added, and the mixture was stirred at 5 to 20°C for 63 hours. The mixture was cooled to 5°C, and 1 N hydrochloric acid (30 ml) was added. Tetrahydrofuran was evaporated away under reduced pressure, and extraction was carried out with ethyl acetate (50 ml). The organic layer was washed with 1 N hydrochloric acid (30 ml), water (10 ml), saturated aqueous solution of sodium hydrogencarbonate (30 ml) and water (10 ml) in order. The solvent was evaporated away under reduced pressure to obtain the Ethyl 3-oxo-4-phenylbutanoate as a pale yellow oil (5.82 g, yield: 86 %).
InChI:InChI=1/C12H14O3/c1-2-15-12(14)9-11(13)8-10-6-4-3-5-7-10/h3-7H,2,8-9H2,1H3
718-08-1 Relevant articles
Deconstructing Noncovalent Kelch-like ECH-Associated Protein 1 (Keap1) Inhibitors into Fragments to Reconstruct New Potent Compounds
Pallesen, Jakob S.,Narayanan, Dilip,Tran, Kim T.,Solbak, Sara M. ?.,Marseglia, Giuseppe,S?rensen, Louis M. E.,H?j, Lars J.,Munafò, Federico,Carmona, Rosa M. C.,Garcia, Anthony D.,Desu, Haritha L.,Brambilla, Roberta,Johansen, Tommy N.,Popowicz, Grzegorz M.,Sattler, Michael,Gajhede, Michael,Bach, Anders
supporting information, p. 4623 - 4661 (2021/05/07)
Targeting the protein-protein interaction (PPI) between nuclear factor erythroid 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) is a potential therapeutic strategy to control diseases involving oxidative stress. Here, six classes of known small-molecule Keap1-Nrf2 PPI inhibitors were dissected into 77 fragments in a fragment-based deconstruction reconstruction (FBDR) study and tested in four orthogonal assays. This gave 17 fragment hits of which six were shown by X-ray crystallography to bind in the Keap1 Kelch binding pocket. Two hits were merged into compound 8 with a 220-380-fold stronger affinity (Ki = 16 μM) relative to the parent fragments. Systematic optimization resulted in several novel analogues with Ki values of 0.04-0.5 μM, binding modes determined by X-ray crystallography, and enhanced microsomal stability. This demonstrates how FBDR can be used to find new fragment hits, elucidate important ligand-protein interactions, and identify new potent inhibitors of the Keap1-Nrf2 PPI.
Discovery of pyrrolo[2,3-d]pyrimidine derivatives as potent Axl inhibitors: Design, synthesis and biological evaluation
Xu, Dandan,Sun, Deqiao,Wang, Wei,Peng, Xia,Zhan, Zhengsheng,Ji, Yinchun,Shen, Yanyan,Geng, Meiyu,Ai, Jing,Duan, Wenhu
, (2021/05/06)
Axl has emerged as an attractive target for cancer therapy due to its strong correlation with tumor growth, metastasis, poor survival, and drug resistance. Herein, we report the design, synthesis and structure-activity relationship (SAR) investigation of a series of pyrrolo[2,3-d]pyrimidine derivatives as new Axl inhibitors. Among them, the most promising compound 13b showed high enzymatic and cellular Axl potencies. Furthermore, 13b possessed preferable pharmacokinetic properties and displayed promising therapeutic effect in BaF3/TEL-Axl xenograft tumor model. Compound 13b may serve as a lead compound for new antitumor drug discovery.
Non-metal Lewis acid-catalyzed cross-Claisen condensation for β-keto esters
Han, Zhengyu,Huang, Hai,Meng, Fuliang,Yang, Zhenkun,Zhang, Tianyu,Zhou, Dapeng
supporting information, p. 9163 - 9166 (2021/11/16)
In this work, we disclose a new catalytic and highly chemoselective cross-Claisen condensation of esters. In the presence of TBSNTf2 as a non-metal Lewis acid, various esters can undergo cross-Claisen condensation to form β-keto esters which are important building blocks. Compared with the traditional Claisen condensation, this process, employing silyl ketene acetals (SKAs) as carbonic nucleophiles to achieve cross-Claisen condensation, requires mild conditions and has good tolerance of functional groups. This journal is
Desymmetrization of meso-dicarbonatecyclohexene with β-Hydrazino carboxylic esters via a Pd-catalyzed allylic substitution cascade
Xu, Kai,Zheng, Yan,Ye, Yong,Liu, Delong,Zhang, Wanbin
supporting information, p. 8836 - 8841 (2020/11/30)
The desymmetrization of meso-dicarbonatecyclohexene with β-hydrazino carboxylic esters has been achieved via a RuPHOX/Pd-catalyzed allylic substitution cascade for the construction of chiral hexahydrocinnoline derivatives with high performance. Mechanistic studies reveal that the reaction exploits a pathway different from that of our previous work and that the first nitrogen nucleophilic process is the rate-determining step. The protocol could be conducted on a gram scale without any loss of catalytic behavior, and the corresponding chiral hexahydrocinnolines can undergo diverse transformations.
718-08-1 Process route
-
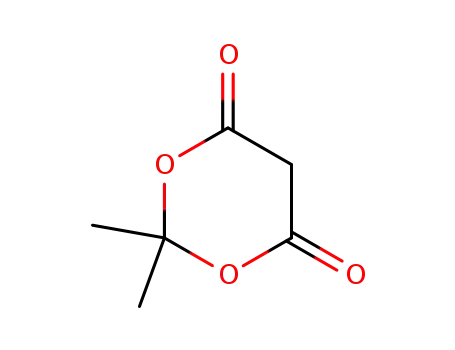
-
2033-24-1
cycl-isopropylidene malonate

-
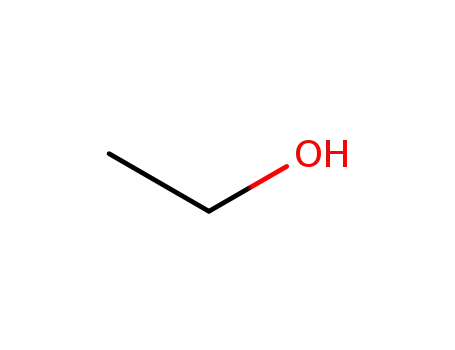
-
64-17-5
ethanol

-
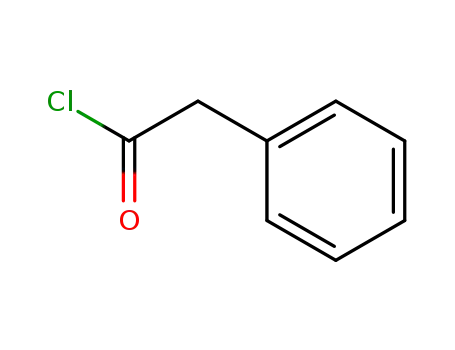
-
103-80-0
phenylacetyl chloride

-
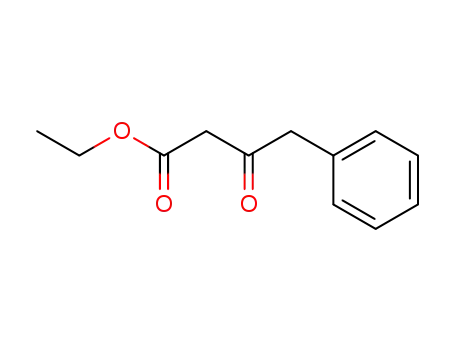
-
718-08-1
ethyl 3-oxo-4-phenylbutyrate
| Conditions | Yield |
|---|---|
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
ethanol;
for 2h;
Heating;
|
88% |
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
for 1.66667h;
ethanol;
for 3h;
Reflux;
|
80% |
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
ethanol;
for 4h;
Further stages.;
Heating;
|
73% |
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
ethanol;
for 2h;
Reflux;
|
69% |
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
ethanol;
for 4h;
Reflux;
|
38% |
|
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
cycl-isopropylidene malonate; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
ethanol;
for 2.5h;
Reflux;
|
33.05 g |
|
cycl-isopropylidene malonate; ethanol; phenylacetyl chloride;
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
Inert atmosphere;
In
ethanol; dichloromethane;
at 80 ℃;
for 4h;
Inert atmosphere;
|
-
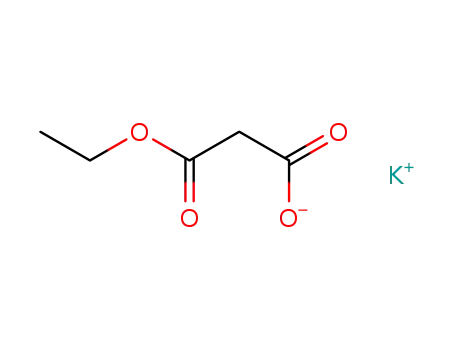
-
6148-64-7
ethyl potassium malonate

-
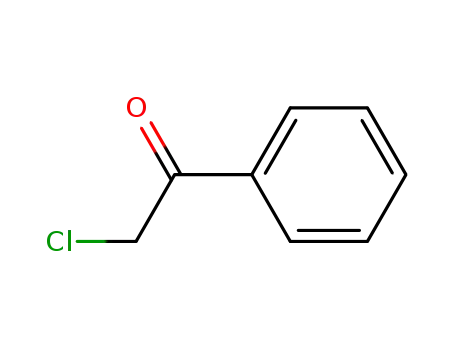
-
532-27-4,108321-66-0,164322-25-2
1-chloroacetophenone

-

-
718-08-1
ethyl 3-oxo-4-phenylbutyrate
| Conditions | Yield |
|---|---|
|
ethyl potassium malonate;
With
triethylamine; magnesium chloride;
In
tetrahydrofuran;
at 5 - 20 ℃;
for 3h;
1-chloroacetophenone;
In
tetrahydrofuran;
at 5 - 20 ℃;
for 63h;
|
86% |
718-08-1 Upstream products
-
412019-21-7
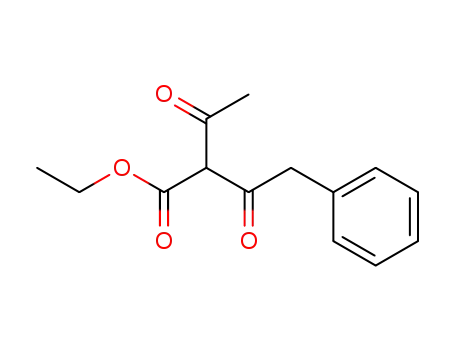
ethyl 2-acetyl-3-oxo-4-phenylbutanoate
-
32864-38-3
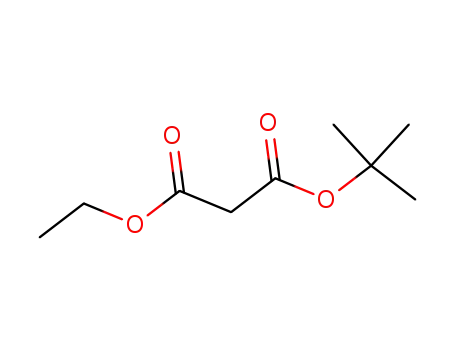
tert-Butyl ethyl malonate
-
103-80-0

phenylacetyl chloride
-
1071-46-1
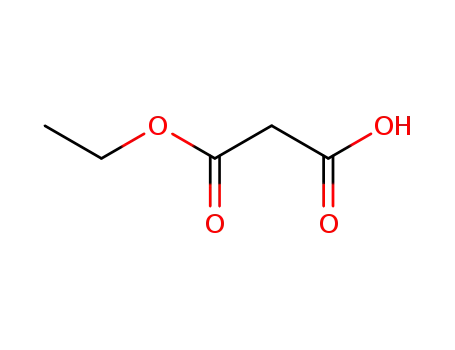
hydrogen ethyl malonate
718-08-1 Downstream products
-
855161-56-7
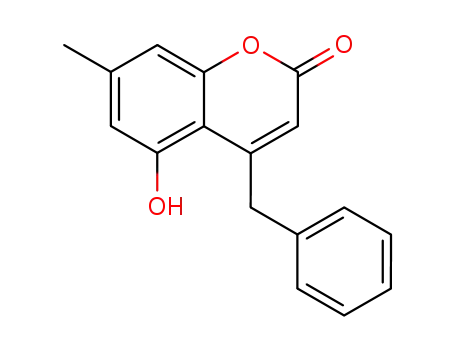
4-benzyl-5-hydroxy-7-methyl-coumarin
-
176519-53-2
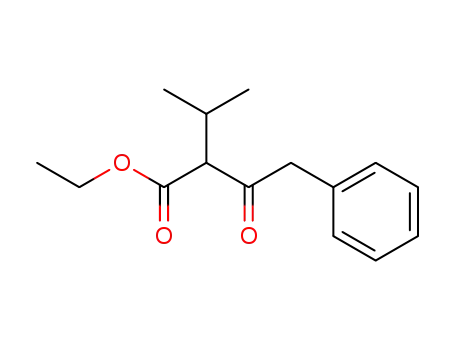
ethyl 2-isopropyl-3-oxo-4-phenylbutanoate
-
102473-93-8
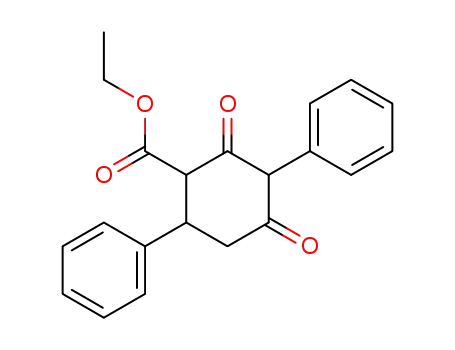
2,4-dioxo-3,6-diphenyl-cyclohexanecarboxylic acid ethyl ester
-
855161-63-6
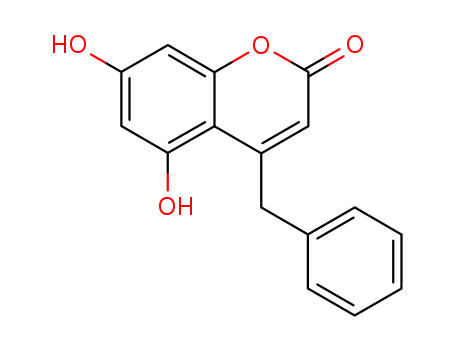
4-benzyl-5,7-dihydroxy-coumarin








