Product Details
123-39-7 Properties
- Molecular Formula:C2H5NO
- Molecular Weight:59.0678
- Appearance/Colour:clear colourless liquid
- Vapor Pressure:0.808mmHg at 25°C
- Melting Point:-3.2 °C
- Refractive Index:n20/D 1.432(lit.)
- Boiling Point:182.5 °C at 760 mmHg
- PKA:16.48±0.23(Predicted)
- Flash Point:81.5 °C
- PSA:29.10000
- Density:0.872 g/cm3
- LogP:0.38900
123-39-7 Usage
InChI:InChI=1/C2H5NO/c1-3-2-4/h2H,1H3,(H,3,4)
123-39-7 Relevant articles
-
Morawetz,Otaki
, p. 463,465 (1963)
-
Highly selective acylation of dimethylamine mediated by oxygen atoms on metallic gold surfaces
Xu, Bingjun,Zhou, Ling,Madix, Robert J.,Friend, Cynthia M.
, p. 394 - 398 (2010)
"Chemical Equation Presented" Completely coupled: The acylation of dimethylamine through coupling to formaldehyde occurs with almost 100 % selectivity at low coverage of adsorbed O atoms on metallic gold and with a low activation energy. Oxygen creates an active intermediate (CH3) 2N(a), which attacks the carbonyl carbon of the aldehyde (see picture). A general mechanistic framework for efficient and selective acylation of amines promoted by Au is established.
Effects of Carbon-Bound Deuterium on the Affinities of Acetaldehyde-1-d and N-Methylformamide-1-d for Solvent Water
Wolfenden, Richard,Kirkman, Sue
, p. 731 - 732 (1983)
With deuterium present in the formyl group, the equilibrium constant for transfer of N-methylformamide from chloroform to D2O at 25 deg C was encanced by factor of 3.1 +/- 0.15 percent, as estimated independently by proton magnetic resonance and by double-labeling experiments in which 14C and 3H were incorporated alternatively into the methyl group.The distribution coefficient of acetaldehyde-1-d between D2O and the vapor phase, on the other hand, differed from that of acetaldehyde by less than 0.5percent.
-
Miyazawa et al.
, p. 408,409,412,414 (1956)
-
-
Carpenter,W.,Bens,E.M.
, p. 59 - 65 (1970)
-
Degradation of gaseous unsymmetrical dimethylhydrazine by vacuum ultraviolet coupled with MnO2
Huang, Yuanzheng,Jia, Ying,Shen, Keke,Hou, Ruomeng,Zhang, Yongyong,Hou, Li'an
, p. 1194 - 1202 (2021)
In this study, α-, β-, and δ-MnO2 were prepared by a uniform hydrothermal method and then coupled with vacuum ultraviolet (VUV) for the degradation of gaseous unsymmetrical dimethylhydrazine (UDMH). The performance in the removal of UDMH, by-product distribution and mechanism were systematically investigated. The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption/desorption, Field Emission Scanning Electron Microscopy (FE-SEM), Raman, thermogravimetry (TG), Fourier-transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) to investigate the factors affecting the catalytic activity. The results showed that O2 and H2O were essential for the removal of UDMH. Photooxidation and ozone catalytic oxidation contribute to the removal and mineralization of UDMH. The integrated process considerably improved the removal and mineralization of UDMH by ozone catalytic oxidation. More reactive oxygen species were generated in the integrated process. The catalytic activity of the prepared catalysts follows the order: δ-MnO2 > α-MnO2 > β-MnO2. δ-MnO2 displayed the highest removal rate of 100% and a CO2 concentration of 42 ppmv. The good performance of δ-MnO2 was mainly attributed to the large number of surface oxygen vacancies.
The Generation of N-Alkylformamides from Synthesis Gas and Ammonia
Knifton, John F.
, p. 1412 - 1414 (1985)
N,N-Dimethylformamide and N-methylformamide have been prepared from synthesis gas plus ammonia via ruthenium 'melt' catalysis.
-
Dunstan,Dymond
, p. 216 (1894)
-
Thermal behavior of ammonium dinitramide and amine nitrate mixtures
Matsunaga, Hiroki,Katoh, Katsumi,Habu, Hiroto,Noda, Masaru,Miyake, Atsumi
, p. 2677 - 2685 (2019)
This paper focuses on the thermal behavior of mixtures of ammonium dinitramide (ADN) and amine nitrates. Because some mixtures of ADN and amine nitrate exhibit low melting points and high-energy content, they represent potential liquid propellants for spacecraft. This study focused on the melting behavior and thermal-decomposition mechanisms in the condensed phase of ADN/amine nitrate mixtures during heating. We measured the melting point and exothermal behavior during constant-rate heating using differential scanning calorimetry and performed thermogravimetry–differential thermal analysis–mass spectrometry (TG–DTA–MS) to analyze the thermal behavior and evolved gases of ADN/amine nitrate mixtures during simultaneous heating to investigate their reaction mechanisms. Results showed that the melting point of ADN was significantly lowered upon the addition of amine nitrate with relatively low molecular volume and low melting point. TG–DTA–MS results showed that the onset temperature of the thermal decomposition of ADN/amine nitrates was similar to that of pure ADN. Furthermore, during thermal decomposition in the condensed phase, ADN produced highly acidic products that promoted exothermic reactions, and we observed the nitration and nitrosation of amines from the dissociation of amine nitrates.
Ru/ceria-catalyzed direct formylation of amines and CO to produce formamides
Wang, Yehong,Zhang, Jian,Chen, Haijun,Zhang, Zhixin,Zhang, Chaofeng,Li, Mingrun,Wang, Feng
, p. 88 - 92 (2017)
We herein report a new strategy of directly converting amines and CO to formamides with 100% atom utilization efficiency. It is suitable for up to 25 amine substrates with no additives. Ru/ceria is found to be an excellent catalyst for this reaction due the efficient co-activation of CO and amine on Ru species.
Development of an empirical kinetic model for sonocatalytic process using neodymium doped zinc oxide nanoparticles
Khataee, Alireza,Vahid, Behrouz,Saadi, Shabnam,Joo, Sang Woo
, p. 146 - 155 (2016)
The degradation of Acid Blue 92 (AB92) solution was investigated using a sonocatalytic process with pure and neodymium (Nd)-doped ZnO nanoparticles. The nanoparticles were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). The 1% Nd-doped ZnO nanoparticles demonstrated the highest sonocatalytic activity for the treatment of AB92 (10 mg/L) with a degradation efficiency (DE%) of 86.20% compared to pure ZnO (62.92%) and sonication (45.73%) after 150 min. The results reveal that the sonocatalytic degradation followed pseudo-first order kinetics. An empirical kinetic model was developed using nonlinear regression analysis to estimate the pseudo-first-order rate constant (kapp) as a function of the operational parameters, including the initial dye concentration (5-25 mg/L), doped-catalyst dosage (0.25-1 g/L), ultrasonic power (150-400 W), and dopant content (1-6% mol). The results from the kinetic model were consistent with the experimental results (R2 = 0.990). Moreover, DE% increases with addition of potassium periodate, peroxydisulfate, and hydrogen peroxide as radical enhancers by generating more free radicals. However, the addition of chloride, carbonate, sulfate, and t-butanol as radical scavengers declines DE%. Suitable reusability of the doped sonocatalyst was proven for several consecutive runs. Some of the produced intermediates were also detected by GC-MS analysis. The phytotoxicity test using Lemna minor (L. minor) plant confirmed the considerable toxicity removal of the AB92 solution after treatment process.
Nitromethane decomposition under high static pressure
Citroni, Margherita,Bini, Roberto,Pagliai, Marco,Cardini, Gianni,Schettino, Vincenzo
, p. 9420 - 9428 (2010)
The room-temperature pressure-induced reaction of nitromethane has been studied by means of infrared spectroscopy in conjunction with ab initio molecular dynamics simulations. The evolution of the IR spectrum during the reaction has been monitored at 32.2 and 35.5 GPa performing the measurements in a diamond anvil cell. The simulations allowed the characterization of the onset of the high-pressure reaction, showing that its mechanism has a complex bimolecular character and involves the formation of the aci-ion of nitromethane. The growth of a three-dimensional disordered polymer has been evidenced both in the experiments and in the simulations. On decompression of the sample, after the reaction, a continuous evolution of the product is observed with a decomposition into smaller molecules. This behavior has been confirmed by the simulations and represents an important novelty in the scene of the known high-pressure reactions of molecular systems. The major reaction product on decompression is N-methylformamide, the smallest molecule containing the peptide bond. The high-pressure reaction of crystalline nitromethane under irradiation at 458 nm was also experimentally studied. The reaction threshold pressure is significantly lowered by the electronic excitation through two-photon absorption, and methanol, not detected in the purely pressure-induced reaction, is formed. The presence of ammonium carbonate is also observed.
Thermal Decomposition of Energetic Materials. 3. Temporal Behaviors of the Rates of Formation of Gaseous Pyrolysis Products from Condensed-Phase Decomposition of 1,3,5-Trinitrohexahydro-s-triazine
Behrens, Richard,Bulusu, Suryanarayana
, p. 8877 - 8891 (1992)
Through the use of simultaneous thermogravimetry modulated beam mass spectrometry (STMBMS) measurements, time-of-flight (TOF) velocity-spectra analysis, and (2)H, (13)C, (15)N and (18)O labeled analogues of 1,3,5-trinitrohexahydro-s-triazine (RDX), the thermal decomposition products of RDX have been identified as H2O, HCN, CO, CH2O, NO, N2O, NH2CHO, NO2, HONO, (CH3)NHCHO, oxy-s-triazine (OST), and 1-nitroso-3,5-dinitrohexahydro-s-triazine (ONDNTA) and all of their gas formation rates have been measured as a function of time.From these results the primary reaction pathways that control the decomposition of RDX in both the solid and liquid phases have been discovered.Four primary reaction pathways control the decomposition of RDX in the liquid phase between 200 and 215 deg C.Two pathways are first-order reactions solely in RDX.One produces predominantly OST, NO, and H2O and accounts for approximately 30percent of the decomposed RDX, and the other produces predominantly N2O and CH2O with smaller amounts of NO2, CO, and NH2CHO and accounts for 10percent of the decomposed RDX.The third pathway consists of formation of ONDNTA by reaction between NO and RDX, followed by the decomposition of ONDNTA to predominantly CH2O and N2O.The fourth reaction pathway consists of decomposition of RDX through reaction with a catalyst that is formed from the decomposition products of previously decomposed RDX.The third and fourth reaction channels each account for approximately 30percent of the decomposed RDX.Experiments with solid-phase RDX have shown that its decomposition rate is very much slower than that of liquid-phase RDX.ONDNTA is the only product that appears to be formed during the early stages of decomposition of RDX in the solid phase.As the solid-phase decomposition progresses, N2O and lesser amounts of CH2O start to evolve and their rates of evolution increase until products associated with the liquid-phase RDX decomposition appear and the rates of gas formation of all products rapidly increase.This behavior strongly suggests the decomposition of solid RDX occurs through formation of ONDNTA within the lattice, the subsequent decomposition of it within the lattice to N2O and CH2O, followed by the dispersion of CH2O in the RDX, leading to its eventual liquefaction and the onset of the liquid-phase decomposition reactions.
-
Winteler et al.
, p. 2370,2376 (1954)
-
N-Heterocyclic carbene catalyzed direct carbonylation of dimethylamine
Li, Xiaonian,Liu, Kun,Xu, Xiaoliang,Ma, Lei,Wang, Hong,Jiang, Dahao,Zhang, Qunfeng,Lu, Chunshan
, p. 7860 - 7862 (2011)
N-Heterocyclic carbene (NHC) catalyzed direct carbonylation of dimethylamine leading to the formation of DMF was successfully accomplished under metal-free conditions. The catalytic efficiency was investigated and the turnover numbers can reach as high as >300. The possible mechanism was also proposed.
Catalytic asymmetric [3+2] cycloaddition of isomünchnones with methyleneindolinones
Feng, Xiaoming,Hu, Xinyue,Lin, Lili,Wang, Kaixuan,Xu, Chaoran,Zhou, Yuqiao
supporting information, p. 8917 - 8920 (2021/09/10)
An efficient enantioselective [3+2] cycloaddition of isomünchnones with methyleneindolinones that are generated by anin situintramolecular addition of the carbonyl group to rhodium carbenes is realized with a chiralN,N′-dioxide/Zn(ii) complex as a Lewis acid. A series of chiral oxa-bridged 3-spiropiperidines are obtained in high yields with excellent dr and excellent ee values.
Evaluation of thioamides, thiolactams and thioureas as hydrogen sulfide (H2S)donors for lowering blood pressure
Zaorska, Ewelina,Hutsch, Tomasz,Gawry?-Kopczyńska, Marta,Ostaszewski, Ryszard,Ufnal, Marcin,Koszelewski, Dominik
supporting information, (2019/04/29)
Hydrogen sulfide (H2S)is a biologically important gaseous molecule that exhibits promising protective effects against a variety of pathological processes. For example, it was recognized as a blood pressure lowering agent. Aligned with the need for easily modifiable platforms for the H2S supply, we report here the preparation and the H2S release kinetics from a series of structurally diversified thioamides, thiolactams and thioureas. Three different thionation methods based on the usage of a phosphorus pentasulfide and Lawesson reagent were applied to prepare the target thioamides and thiolactams. Furthermore, obtained H2S donors were evaluated both in in vivo and in vitro studies. The kinetic parameters of the liberating H2S was determined and compared with NaHS and GYY4137 using two different detection technics i.e.; fluorescence labeling 7-azido-4-methyl-2H-chromen-2-one and 5,5‘-dithiobis (2-nitrobenzoic acid), sulfhydryl probe, also known as the Ellman's reagent. We have proved that the amount of releasing H2S from these compounds is controllable through structural modifications. Finally, the present study shows a hypotensive response to an intravenous administration of the developed donors in the anesthetized rats.
123-39-7 Process route
-
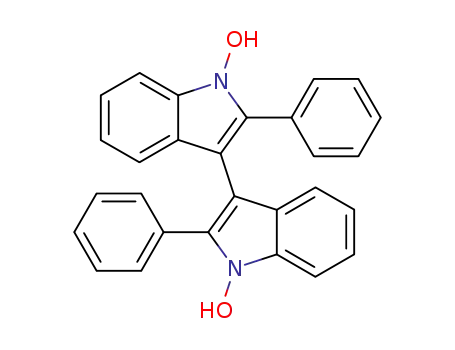
![2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide](/upload/2023/2/329db51f-0979-4396-ae7c-21941536307f.png)
-
2196-95-4,17213-48-8
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide

-
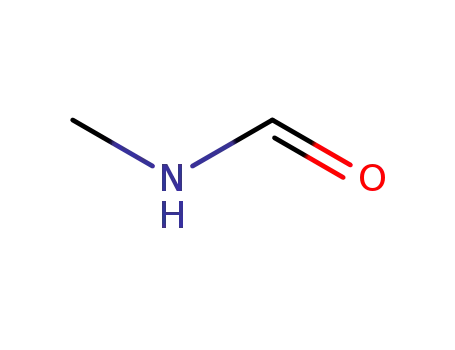
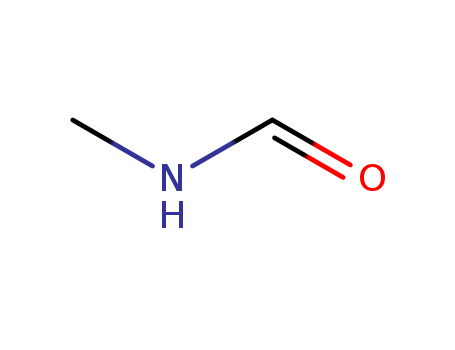
-
123-39-7
N-Methylformamide

-
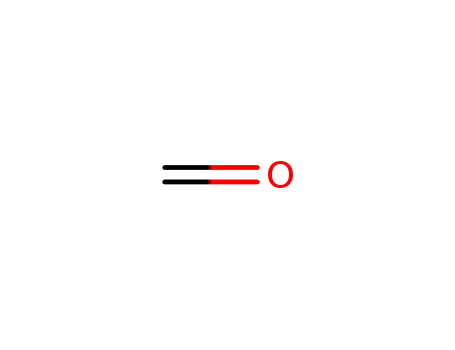
-
50-00-0,30525-89-4,61233-19-0
formaldehyd

-
-
5169-64-2
1,1'-dihydroxy-2,2'-diphenyl-3,3'-biindole

-
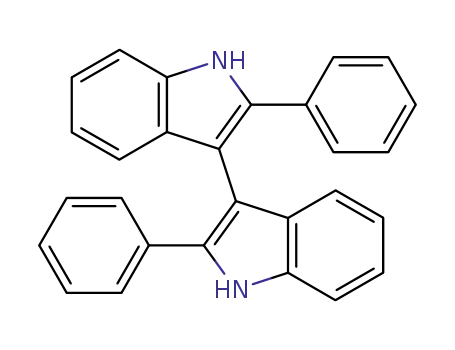
-
2415-33-0
2,2'-diphenyl-1H,1'H-3,3'-biindole
| Conditions | Yield |
|---|---|
|
With
N,N-dimethyl-formamide;
at 140 ℃;
for 14h;
|
35% 45% |
-
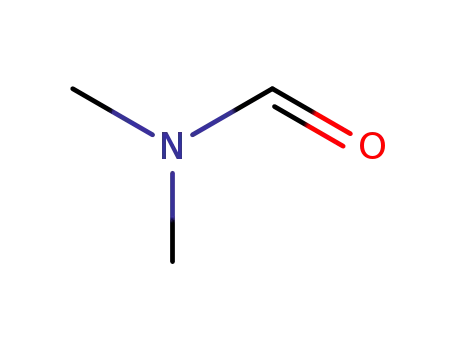
-
68-12-2,33513-42-7
N,N-dimethyl-formamide

-
![2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide](/upload/2023/2/329db51f-0979-4396-ae7c-21941536307f.png)
-
2196-95-4,17213-48-8
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide

-

-
123-39-7
N-Methylformamide

-
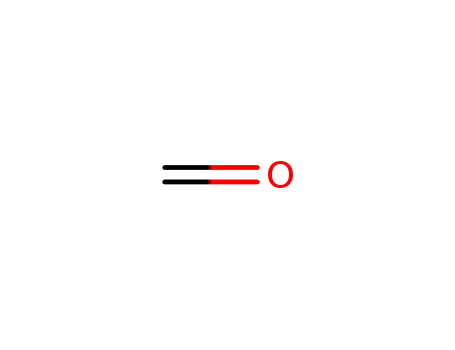
-
50-00-0,30525-89-4,61233-19-0
formaldehyd

-

-
5169-64-2
1,1'-dihydroxy-2,2'-diphenyl-3,3'-biindole

-

-
2415-33-0
2,2'-diphenyl-1H,1'H-3,3'-biindole
| Conditions | Yield |
|---|---|
|
In
benzene;
at 140 ℃;
for 14h;
Product distribution;
|
45% 35% |
|
In
benzene;
at 140 ℃;
for 14h;
|
45% 35% |
123-39-7 Upstream products
-
593-75-9
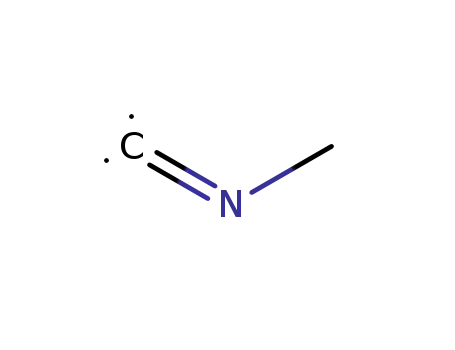
methyl isocyanate
-
100-97-0
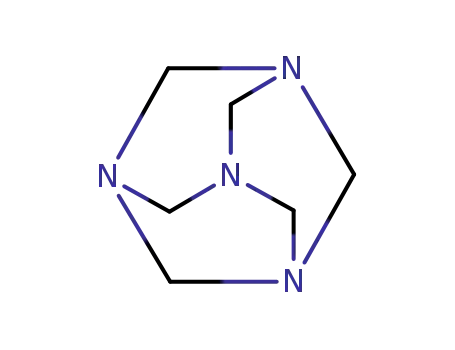
hexamethylenetetramine
-
77287-34-4
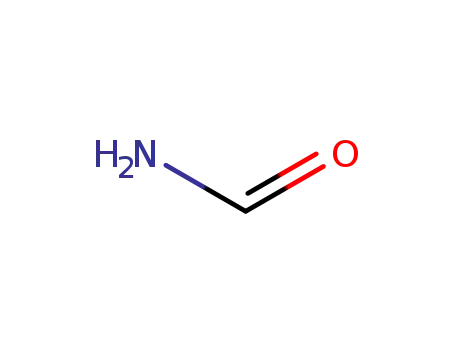
formamide
-
21802-57-3
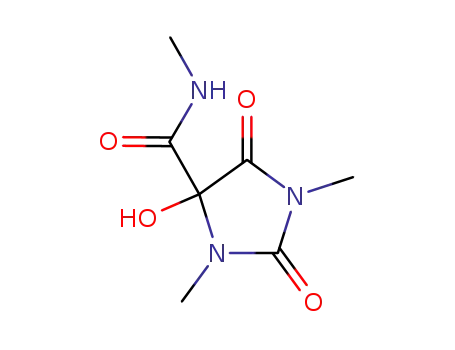
4-hydroxy-1,3-dimethyl-2,5-dioxo-imidazolidine-4-carboxylic acid methylamide
123-39-7 Downstream products
-
25115-67-7
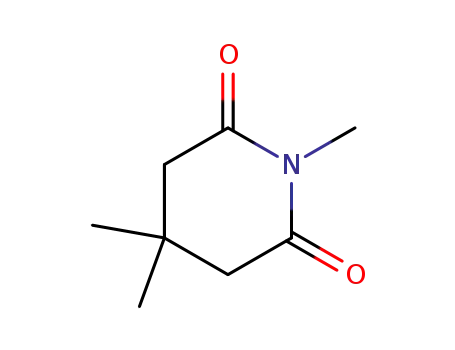
N,3,3-trimethylglutarimide
-
61414-22-0
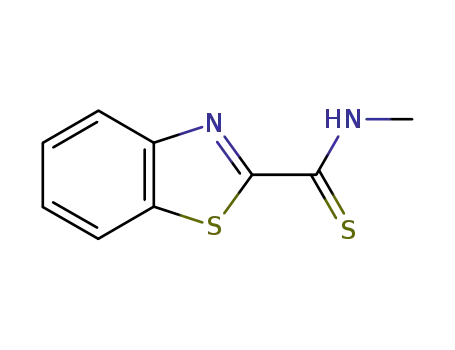
N-methyl-1,3-benzothiazole-2-carbothioamide
-
100127-74-0
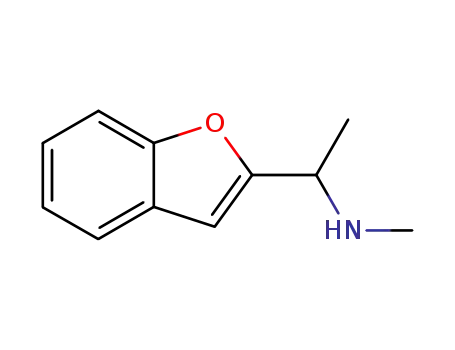
(1-benzofuran-2-yl-ethyl)-methyl-amine
-
58-08-2
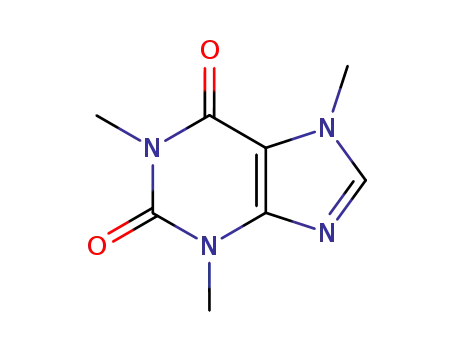
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione








