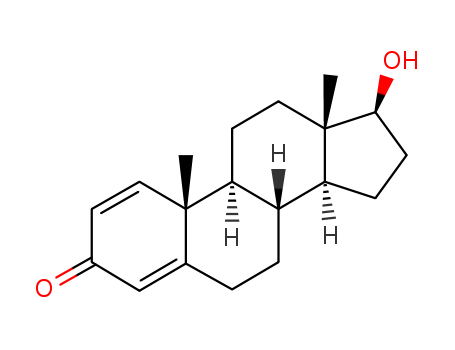
Product Details
846-48-0 Properties
- Molecular Formula:C19H26O2
- Molecular Weight:286.414
- Appearance/Colour:crystalline solid
- Vapor Pressure:2.04E-09mmHg at 25°C
- Melting Point:164-166 °C
- Refractive Index:1.577
- Boiling Point:435.6 °C at 760 mmHg
- PKA:15.05±0.60(Predicted)
- Flash Point:185.8 °C
- PSA:37.30000
- Density:1.14 g/cm3
- LogP:3.65520
846-48-0 Usage
Description
Boldenone is the chemical precursor of its endecylenate ester prodrug boldenone undecylenate – which is used extensively as a steroid for animals, mainly horses and cattle, under the brand name Equipoise, among others. For this application, it is injected to improve the weight, hair coat, appetite, and general physical condition of horses affected by disease, anorexia, or overwork. It is also used as an illegal doping agent for human athletes. As a derivative of testosterone, it retains its anabolic strength, but exhibits reduced androgenic effects.
Sources
https://en.wikipedia.org/wiki/Boldenone_undecylenate https://en.wikipedia.org/wiki/Boldenone https://www.drugs.com/vet/equipoise.html https://www.steroidal.com/steroid-profiles/equipoise/ https://www.evolutionary.org/equipoise-boldenone-undecylenate
Description
Boldenone (Item No. 15432) is an analytical reference standard categorized as an anabolic androgenic steroid. Anabolic steroids, including boldenone, have been used to enhance physical performance in athletes. Boldenone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications.
Chemical Properties
Crystalline Solid
Uses
Boldenone is an anabolic steroid. Controlled substance.
Uses
Controlled substance. Anabolic steroid
Definition
ChEBI: An 3-oxo-Delta1,Delta4-steroid substituted by an oxo group at position 3 and a beta-hydroxy group at position 17. It is an anabolic androgenic steroid that has been eveloped for veterinary use.
Brand name
Equipoise [Veterinary] (Fort Dodge Animal Health).
Synthesis
Boldenone is obtained by reacting Testosterone with tert-butyldimethylsilyl chloride in the presence of DDQ. Experimental Procedure: 29.0 g Testosterone was dissolved in 220 ml dioxan/THF 8:2 and cooled to 0°C on ice. After cooling down a solution of tert-butyldimethylsilyl chloride (46.0 g in 100 ml dioxan/THF 8:2) was added dropwise and stirred for 90 min at 0°C. Then a suspension of 32.0 g of DDQ in 200 ml dioxan/THF 8:2 was added in 4 equal portions over a period of 4 h. The reaction mixture was stirred for additional 12 h within coming from 0°C to room temperature. The resulting suspension was then filtered over Celite and rinsed with 300 ml THF. The filtrate was evaporated and brown oil was obtained. The oil was diluted in 1500 ml DCM and washed with 500 ml of 5% aqueous sodium hydroxide. The yellow organic phase was washed with 400 ml water and 400 ml saturated sodium chloride solution, before drying over magnesium sulfate. After evaporation, the crude product was chromatographed on silica gel by using hexane-ethyl acetate (3:7), followed by a recrystallization in ethyl acetate. 16.50 g of Boldenone was obtained as an amber solid (58%).
InChI:InChI=1/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,11,14-17,21H,3-6,8,10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1
846-48-0 Relevant articles
Microbial transformation of epiandrosterone by Aspergillus sydowii
Yildirim, Kudret,Kuru, Ali
, p. 718 - 721 (2016/12/30)
Incubation of epiandrosterone with Aspergillus sydowii MRC 200653 afforded ten metabolites. The fungal dehydrogenation of epiandrosterone is reported for the first time. The formation of the major metabolite, 6?-hydroxyandrost-4-ene-3,17-dione, involved first dehydrogenation to give a 4-ene and then hydroxylation at C-6?. Small amounts of the substrate were hydroxylated at C-1α, C-7α, C-7β and C-11α.
Old yellow enzyme-catalyzed dehydrogenation of saturated ketones
Schittmayer, Matthias,Glieder, Anton,Uhl, Michael K.,Winkler, Andreas,Zach, Simone,Schrittwieser, Joerg H.,Kroutil, Wolfgang,MacHeroux, Peter,Gruber, Karl,Kambourakis, Spiros,Rozzell, J. David,Winkler, Margit
experimental part, p. 268 - 274 (2011/04/22)
Enzymes from extremophiles have always been of great interest for biotechnology because of their ruggedness against various stress factors. We have isolated, cloned, heterologously expressed and characterized a thermostable old yellow enzyme (OYE) from Geobacillus kaustophilus. In addition to the expected 'enone' reduction, GkOYE also catalyzes the reverse reaction, i.e., the desaturation of C-C bonds adjacent to a carbonyl to give the corresponding α,β-unsaturated ketone. The reaction proceeds at the expense of molecular oxygen without the need for a nicotinamide cofactor and represents an environmentally benign alternative to known chemical dehydrogenation methods.
Endogenous boldenone-formation in cattle: Alternative invertebrate organisms to elucidate the enzymatic pathway and the potential role of edible fungi on cattle's feed
Verheyden,Noppe,Zorn,Van Immerseel,Bussche, J. Vanden,Wille,Bekaert,Janssen,De Brabander,Vanhaecke
experimental part, p. 161 - 170 (2011/03/19)
Although β-boldenone (bBol) used to be a marker of illegal steroid administration in calves, its endogenous formation has recently been demonstrated in these vertebrates. However, research on the pathway leading to bBol remains scarce. This study shows the usefulness of in vivo invertebrate models as alternatives to vertebrate animal experiments, using Neomysis integer and Lucilia sericata. In accordance with vertebrates, androstenedione (AED) was the main metabolite of β-testosterone (bT) produced by these invertebrates, and bBol was also frequently detected. Moreover, in vitro experiments using feed-borne fungi and microsomes were useful to perform the pathway from bT to bBol. Even the conversion of phytosterols into steroids was shown in vitro. Both in vivo and in vitro, the conversion of bT into bBol could be demonstrated in this study. Metabolism of phytosterols by feed-borne fungi may be of particular importance to explain the endogenous bBol-formation by cattle. To the best of our knowledge, it is the first time the latter pathway is described in literature.
A practical Δ1-dehydrogenation of Δ4-3-keto-steroids with DDQ in the presence of TBDMSCl at room temperature
Chen, Kaixiong,Liu, Chang,Deng, Le,Xu, Guangyu
experimental part, p. 513 - 516 (2010/06/21)
A mild and efficient Δ1-dehydrogenation of Δ4-3-keto-steroids with DDQ in the presence of tertbutyldimethylchlorosilane at room temperature was developed.
846-48-0 Process route
-
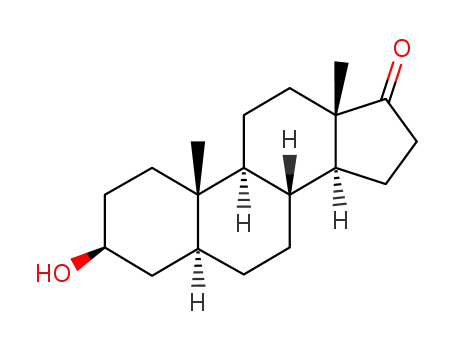
-
481-29-8
Epiandrosterone

-
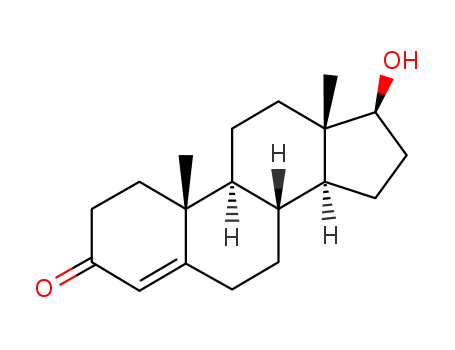
-
58-22-0
testosterone

-
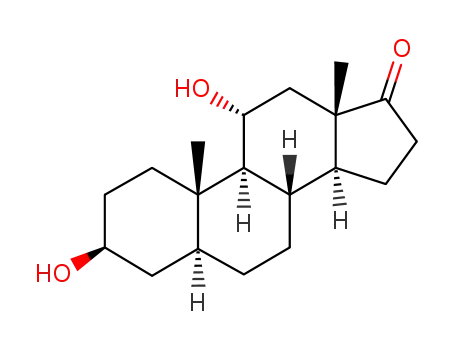
-
25848-75-3
3β,11α-dihydroxy-5α-androstan-17-one

-
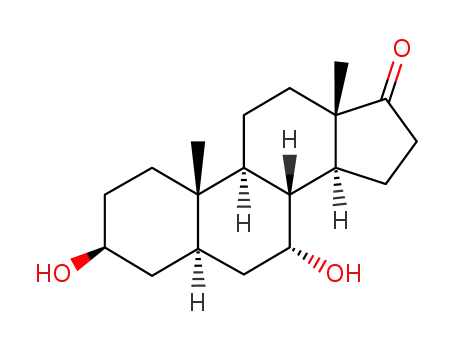
-
25848-68-4
3beta-7alpha-Dihydroxy-5alpha-androstane-17-one

-
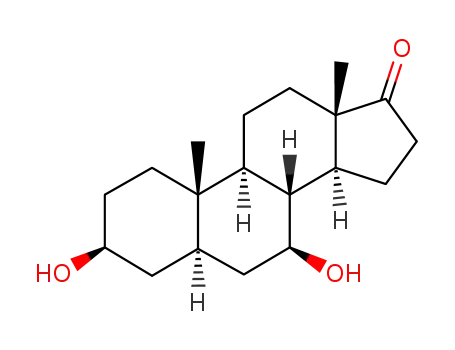
-
25848-69-5
3beta-7beta-Dihydroxy-5alpha-androstane-17-one

-
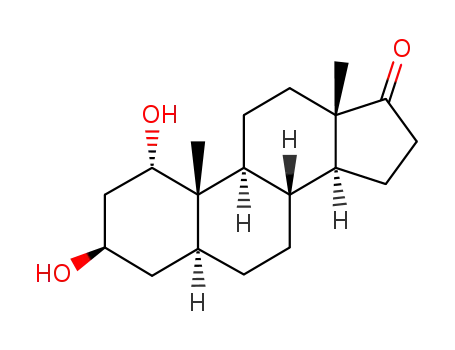
-
2260-01-7
1α,3β-dihydroxy-5α-androstan-17-one

-
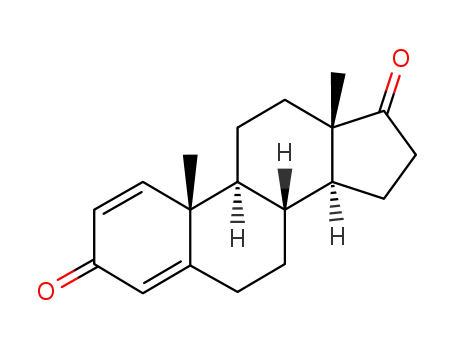
-
897-06-3
Androsta-1,4-diene-3,17-dione

-
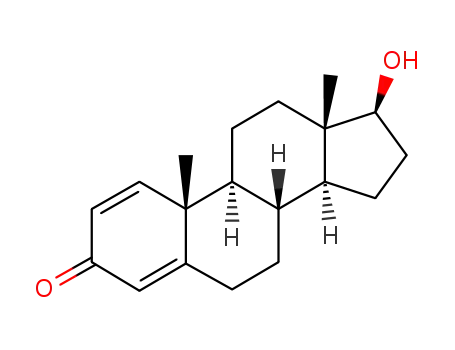
-
846-48-0,361432-76-0
1-dehydrotestosterone

-
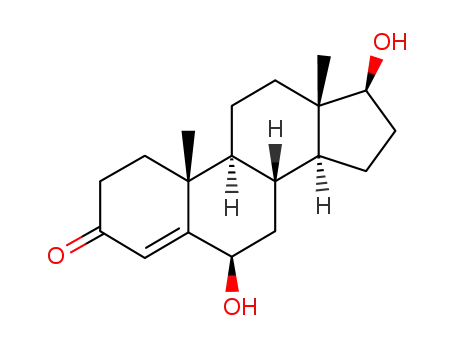
-
62-99-7,2944-87-8,34096-63-4,34442-08-5,145772-79-8
6β-hydroxytestosterone

-
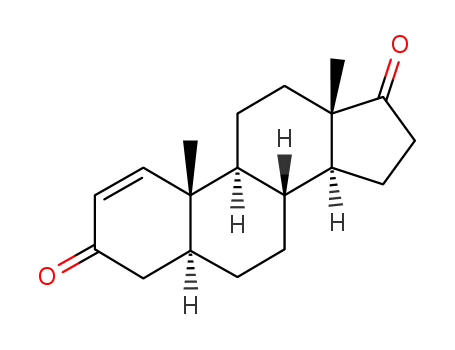
-
571-40-4
5α-androst-1-ene-3,17-dione

-
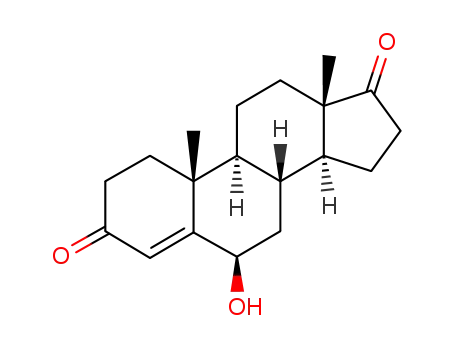
-
63-00-3
6β-hydroxy-4-androstene-3,17-dione
| Conditions | Yield |
|---|---|
|
With
malt extract; disodium hydrogenphosphate; calcium(II) chloride dihydrate; magnesium(II) chloride hexahydrate; strontium (III) chloride hexahydrate; potassium chloride; boric acid; sodium hydrogencarbonate; sodium sulfate; sodium chloride; potassium bromide; potassium hydroxide;
In
water; N,N-dimethyl-formamide;
at 32 ℃;
for 168h;
pH=8;
Enzymatic reaction;
|
2% 5% 2% 3% 32% 2% 2% 5% 3% 2% |
-
-
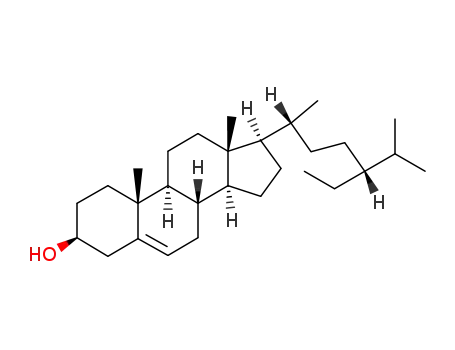
83-46-5
β-sitosterol

-

-
58-22-0
testosterone

-

-
846-48-0,361432-76-0
1-dehydrotestosterone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: unidentified fungal species isolated from grinded corn / water / 1 h / 37 °C / pH 7 / aq. phosphate buffer; Microbiological reaction; Enzymatic reaction
2: unidentified fungal species isolated from grinded corn / water / 1 h / 37 °C / pH 7 / aq. phosphate buffer; Microbiological reaction; Enzymatic reaction
3: unidentified fungal species isolated from grinded corn / water / 1 h / 37 °C / pH 7 / aq. phosphate buffer; Microbiological reaction; Enzymatic reaction
With
unidentified fungal species isolated from grinded corn;
In
water;
|
846-48-0 Upstream products
-
108-75-8
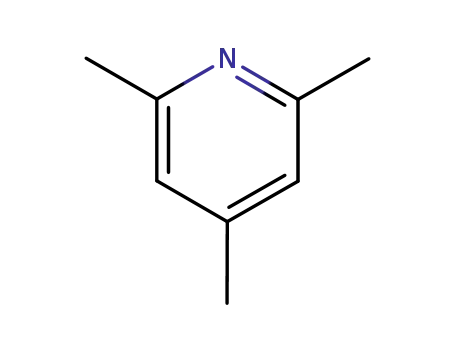
2,4,6-trimethyl-pyridine
-
119369-73-2
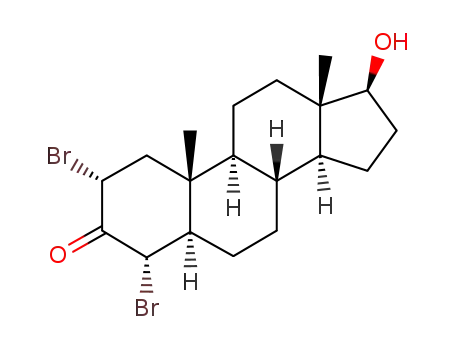
2α,4α-dibromo-17β-hydroxy-5α-androstan-3-one
-
58-22-0

testosterone
-
57-83-0
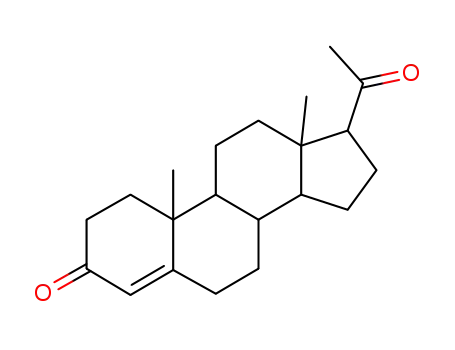
progesterone
846-48-0 Downstream products
-
897-06-3

Androsta-1,4-diene-3,17-dione
-
19041-66-8
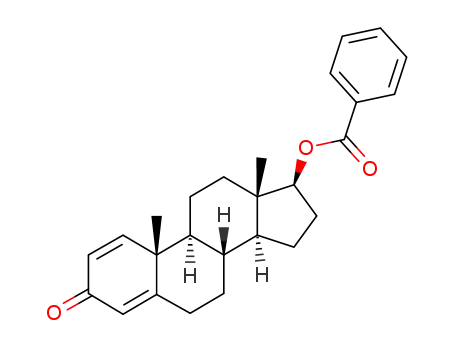
boldenone benzoate
-
50-28-2
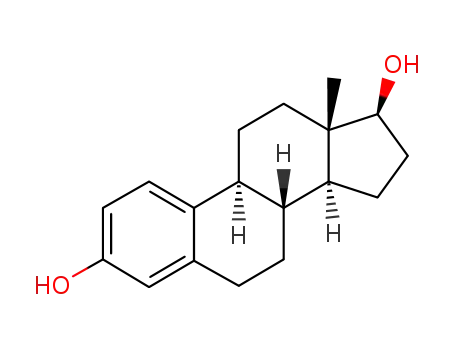
estradiol
-
122370-91-6
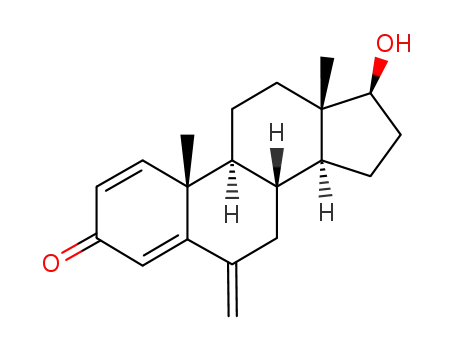
17β-hydroxy-6-methylen- androsta-1,4-dien-3-one








