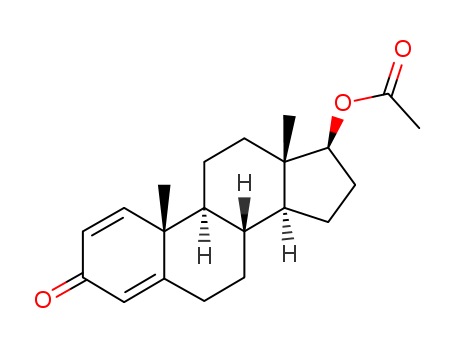
Product Details
2363-59-9 Properties
- Molecular Formula:C21H28O3
- Molecular Weight:328.452
- Vapor Pressure:4.58E-08mmHg at 25°C
- Melting Point:149-151 °C
- Refractive Index:1.555
- Boiling Point:443.6 °C at 760 mmHg
- Flash Point:192.7 °C
- PSA:43.37000
- Density:1.13 g/cm3
- LogP:4.22600
2363-59-9 Usage
Description
Boldenone 17-acetate is a white or white solid powder, belongs to the hormone class.
Uses
The parent of 17-acetate bodanone is a derivative of testosterone and thus inherits most of its properties, such as androgenic ability and protein synthesis.
InChI:InChI=1/C21H28O3/c1-13(22)24-19-7-6-17-16-5-4-14-12-15(23)8-10-20(14,2)18(16)9-11-21(17,19)3/h8,10,12,16-19H,4-7,9,11H2,1-3H3
2363-59-9 Relevant articles
STEROID HOMOLOG CONTAINING A PYRAZOLE NUCLEUS.
VIDA,GUT
, p. 792 - 793 (1963)
-
Use of [1,2-3H] Testosterone in 5- Reductase Enzymatic Activity Dosing in Dermal Fibroblast Cultures from Polycystic Ovarian Patients
Matei, L.,Postolache, C.,Fugaru, V.,Condac, E.,Dinichiotu, A.,Costache, M.
, p. 1055 - 1056 (2007/10/03)
-
2-iodo-3-keto-Δ4 steroids, process for their production, as well as their further processing
-
, (2008/06/13)
The new intermediate products of general formula I STR1 in which R1 stands for a hydrogen atom or a straight-chain or branched alkyl group with 1 to 4 carbon atoms, R2 stands for a hydrogen atom or a methyl group, R3 stands for a hydrogen atom, R4 stands for an acyloxy group with 1 to 4 carbon atoms in the acyl radical or R3 and R4 together stand for a keto-oxygen atom, are suitable in an excellent way for introducing a Δ1 double bond in the steroid skelton with the simultaneous presence of a Δ4 double bond, as well as a saturated carbonyl group, by clevage of hydrogen iodide with a base in an amidic solvent at elevated temperature. If R2 stands for a hydrogen atom, the A-ring is aromatized after the hydrogen iodide cleavage. For the production of a new intermediate products, special iodization processes, which partially also allow a stereoselective control of the iodization, are used.
2363-59-9 Process route
-
-
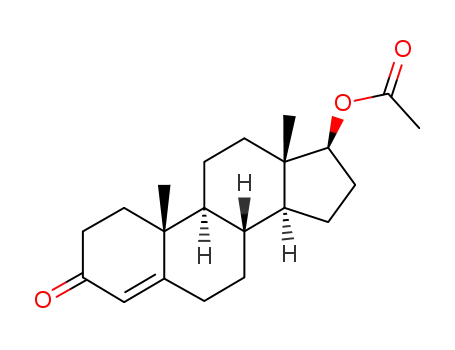
1045-69-8
testosterone acetate

-
-
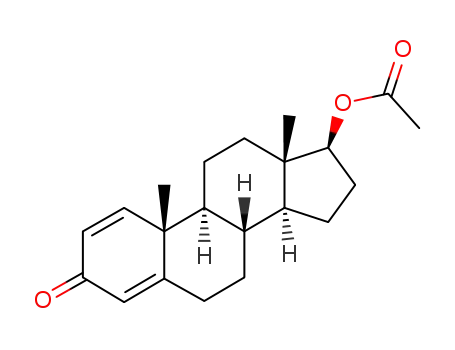
2363-59-9
17β-acetoxy-androsta-1,4-dien-3-one
| Conditions | Yield |
|---|---|
|
With
thallium(III) acetate;
In
acetic acid;
|
|
|
With
2,6-dichloro-3,5-dicyano-1,4-benzoquinone;
|
-
![Acetic acid (8R,9S,10R,13S,14S,17S)-6-bromo-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl ester](/upload/2023/2/100e749d-77b9-473f-bce1-c446729f105b.png)
-
Acetic acid (8R,9S,10R,13S,14S,17S)-6-bromo-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl ester

-

-
2363-59-9
17β-acetoxy-androsta-1,4-dien-3-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: Zn / aq. ethanol
2: (i) (UV-irradiation), EtOH, (ii) KOH, MeOH, (iii) /BRN= 385737/, Py
With
zinc;
In
ethanol;
|
2363-59-9 Upstream products
-
108-75-8
2,4,6-trimethyl-pyridine
-
82926-51-0
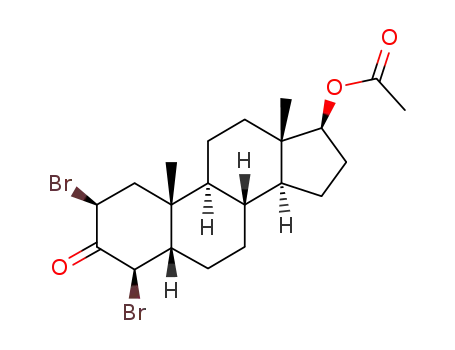
17β-acetoxy-2β,4β-dibromo-5β-androstan-3-one
-
15359-18-9
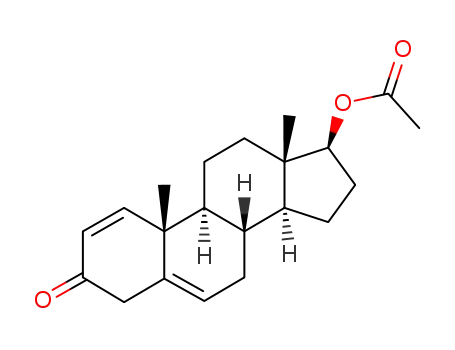
17β-Acetoxy-3-oxo-Δ1,5-androstadien
-
108-24-7
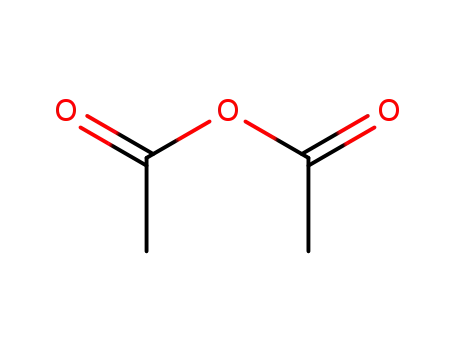
acetic anhydride
2363-59-9 Downstream products
-
2456-11-3
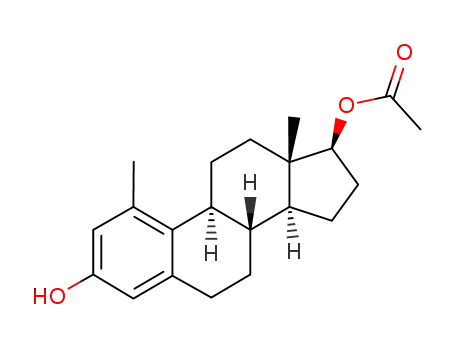
1-Methyl-17-o-acetyl-oestradiol
-
2383-80-4
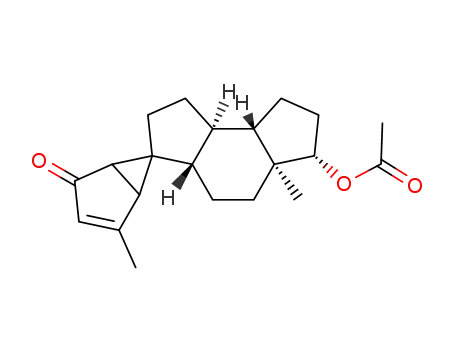
(1Ξ,6Ξ,3'aS)-6't-acetoxy-4,5'a-dimethyl-(3'ar,5'at,8'ac,8'bt)-decahydro-spiro[bicyclo[3.1.0]hex-3-ene-6,3'-As-indacen]-2-one
-
2363-60-2
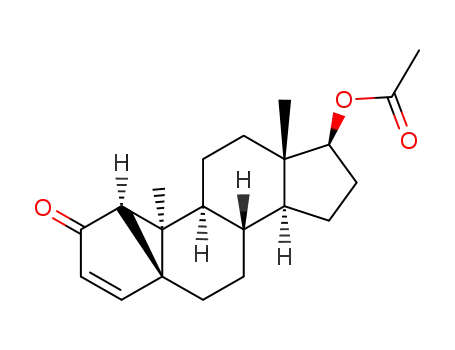
17β-acetoxy-1β,5β-cyclo-10α-androst-3-en-2-one
-
1045-69-8

testosterone acetate








