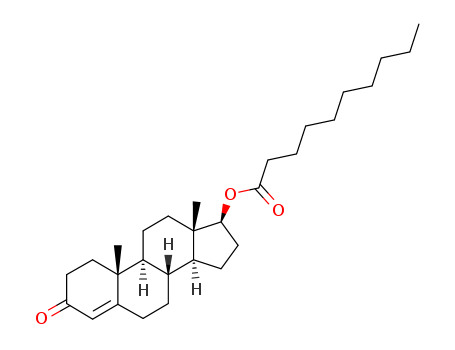
Testosterone Decanoate 5721-91-5
- CasNo:5721-91-5
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
5721-91-5 Properties
- Molecular Formula:C29H46O3
- Molecular Weight:442.682
- Appearance/Colour:White or yellow-white crystalline powder
- Vapor Pressure:1.08E-11mmHg at 25°C
- Melting Point:38 °C
- Refractive Index:1.523
- Boiling Point:539.2 °C at 760 mmHg
- Flash Point:226.4 °C
- PSA:43.37000
- Density:1.04 g/cm3
- LogP:7.57080
5721-91-5 Usage
Description
Testosterone Decanoate is one of the many esterified forms of testosterone and is a long form of the testosterone as it offers a long half life after administration. This is actually an androgen and anabolic steroid and a testosterone ester, a potent long acting injectable male synthetic hormone. Testosterone Decanoate is second longest acting form of testosterone after Testosterone Undecanoate. As with any other form of testosterone, the only difference between them is in the half life (the release time of the hormone after it got administered).
Chemical Properties
White or almost white powder.
Uses
Testosterone decanoate is an androgen that mainly used by men to maintain the second sexual sign, sexual desire and male psychology. It is an steroid indicated for testosterone replacement therapy in sterilization, missing testicle, hypopituitarism, and osteoporosis.
InChI:InChI=1/C29H46O3/c1-4-5-6-7-8-9-10-11-27(31)32-26-15-14-24-23-13-12-21-20-22(30)16-18-28(21,2)25(23)17-19-29(24,26)3/h20,23-26H,4-19H2,1-3H3/t23-,24-,25-,26-,28-,29-/m0/s1
5721-91-5 Relevant articles
Highly efficient, solvent-free esterification of testosterone promoted by a recyclable polymer-supported tosylic acid catalyst under microwave irradiation
Borowiecki, Pawe?,Kraszewski, Maciej
, p. 288 - 305 (2020/02/13)
Although the classical acylation of testosterone clearly benefits from a broad substrate scope and available catalysts, the requirement of hazardous reagents and the high waste production are its drawbacks. To optimize the process efficiency as well as minimize the environmental impact, we decided to develop a novel method of testosterone esters synthesis, which relies on the usage of recyclable heterogeneous polymer-supported tosylic acid catalyst and microwave-assistance effect in a non-solvent system. Under the established MW-conditions, the acceleration of the process rate was so efficient that the reaction completed within 2.5 min, thus affording the desired esters in the 33–96% yield range without using a work-up procedure. Furthermore, the elaborated catalytic system could be recycled for at least 2 runs not only without a loss of the products yield, but unexpectedly with significant improvement of the reaction efficiency, which may indicate that the reduction of the catalyst loading is possible. We believe that this finding constitutes a very good starting-point for further optimization of the studied process.
Long-range effect of 17-substituents in 3-oxo steroids on 4,5-double bond hydrogenation
Sidova, Romana,Stransky, Karel,Kasal, Alexander,Slavikova, Barbora,Kohout, Ladislav
, p. 1528 - 1542 (2007/10/03)
The long-range effect of substituents in the 17-position on the hydrogenation of double bond of the steroidal Δ4-3-ketones in acetic acid on a platinum catalyst is described in a series of testosterone (1) and epitestosterone (5) esters with carboxylic acids of varying alkyl chain length. The ratio 5α-to 5β-products is affected by the nature of substituents in the position 17.
5721-91-5 Process route
-
-
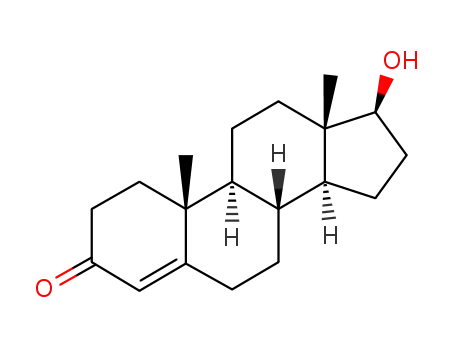
58-22-0
testosterone

-
-
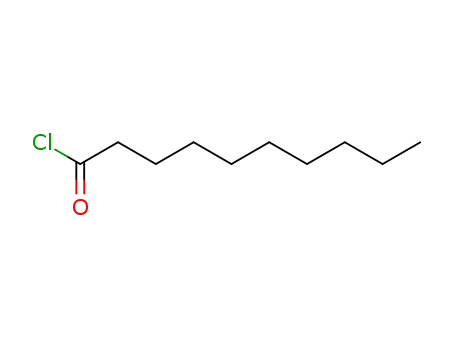
112-13-0
n-decanoyl chloride

-
-
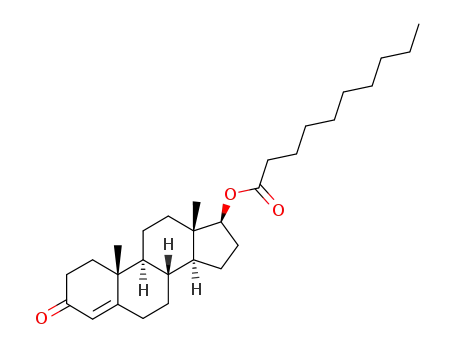
5721-91-5
3-oxoandrost-4-en-17β-yl decanoate
| Conditions | Yield |
|---|---|
|
With
pyridine;
Ambient temperature;
|
85% |
|
With
immobilized p-toluenesulfonic acid polymer bound macroporous;
In
neat (no solvent);
at 100 ℃;
for 0.0416667h;
Microwave irradiation;
Sealed tube;
Green chemistry;
|
66% |
-
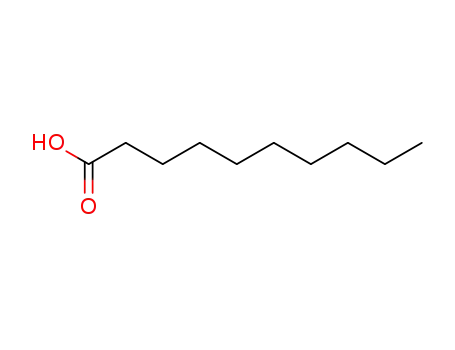
-
334-48-5
1-decanoic acid

-

-
58-22-0
testosterone

-

-
5721-91-5
3-oxoandrost-4-en-17β-yl decanoate
| Conditions | Yield |
|---|---|
|
at 200 ℃;
|
5721-91-5 Upstream products
-
334-48-5
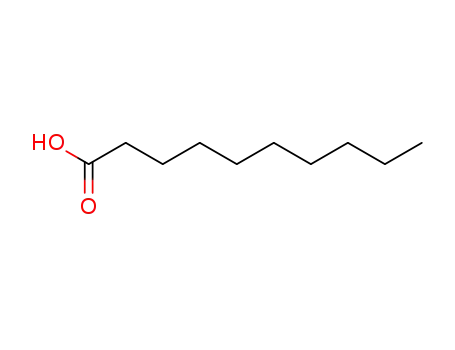
1-decanoic acid
-
58-22-0

testosterone
-
112-13-0

n-decanoyl chloride
-
481-30-1
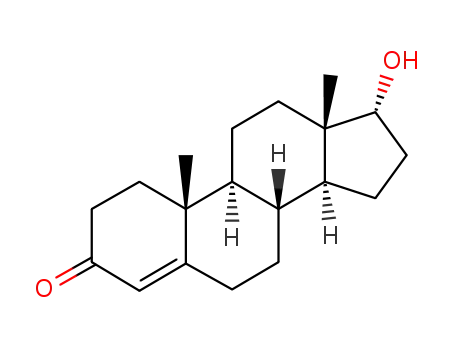
epitestosterone








