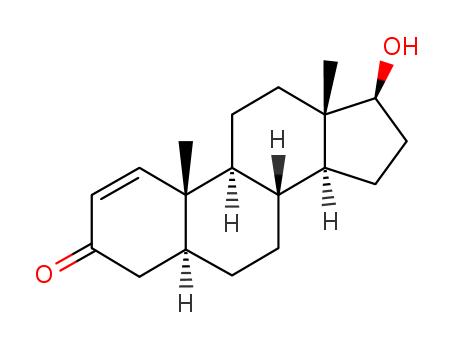
Product Details
65-06-5 Properties
- Molecular Formula:C19H28 O2
- Molecular Weight:288.43
- Vapor Pressure:7.84E-09mmHg at 25°C
- Melting Point:75-85°C
- Refractive Index:1.549
- Boiling Point:120°C 0,5mm
- PKA:15.08±0.60(Predicted)
- Flash Point:179.5oC
- PSA:37.30000
- Density:1.1561
- LogP:3.73510
65-06-5 Usage
Description
1-Testosterone (Item No. 24079) is an analytical reference standard categorized as an anabolic androgenic steroid. Anabolic steroids, including 1-testosterone, have been used to enhance physical performance in racehorses and athletes. 1-Testosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications.
Chemical Properties
White Solid
Uses
1-Testosterone is a potent androgen with anabolic properties. Since the begining of the year 2005, the use of steroid precursors (prohormones) is illegal in the United States.
InChI:InChI=1/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,12,14-17,21H,3-6,8,10-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1
65-06-5 Relevant articles
ANTI-CANCER NUCLEAR HORMONE RECEPTOR-TARGETING COMPOUNDS
-
Page/Page column 147-149, (2021/05/21)
The disclosure relates to anti-cancer compounds which are anti-cancer PARP inhibitors of formula Al, A2, A3 or A4 conjugated by a linker to a steroid, whereby the steroid targets the conjugate to the nucleus, as well as to methods for their preparation and use. (I)
An efficient synthesis of 5α-androst-1-ene-3,17-dione
Zhang, Huyue,Qiu, Zhuibai
, p. 1088 - 1090 (2007/10/03)
5α-Androst-1-ene-3,17-dione (5) as a prodrug of 1-testosterone (4) was prepared in four steps from 17β-Acetoxy-5α-androstan-3-one (stanolone acetate) (1) in high yield. Thus, stanolone acetate (1) was brominated in the presence of hydrogen chloride in acetic acid to give 17β-acetoxy-2-bromo-5α-androstan-3-one (2), which underwent dehydrobromination using lithium carbonate as base with lithium bromide as an additive to give 17β-acetoxy-5α-androst-1-en-3-one (3) in almost quantitative yield with 97% of purity. Compound (3) was hydrolyzed with sodium hydroxide to give 17β-hydroxy-5α-androst-1-en-3-one (4,1-testosterone), which was oxidized with chromium trioxide to afford 5α-androst-1-ene-3,17-dione (5). The overall yield of 5 was 78.2% with purity of 99%. In this method, the formation of 4-ene was diminished when 1-ene was introduced, and its mechanism was also discussed.
HIO3 and I2O5: Mild and selective alternative reagents to IBX for the dehydrogenation of aldehydes and ketones
Nicolaou,Montagnon, Tamsyn,Baran, Phil S.
, p. 1386 - 1389 (2007/10/03)
Economic and convenient: Iodic acid (1) and iodine pentoxide (2) form complexes 3 and 4, respectively, with DMSO when heated at 80°C for 1 h. The complexes are efficient agents for the dehydrogenation of ketones and aldehydes at 45-65°C. X-ray crystallographic analysis (see picture) shows that the iodine pentoxide. DMSO complex 4 self-assembles into a remarkable helix in the solid state.
A facile synthesis of 1β-hydroxytestosterone
Sharma, P K,Akhila, A
, p. 554 - 556 (2007/10/02)
A convenient synthesis of androst-4-en-1β,17β-diol-3-one (1β-hydroxytestosterone, 1) from 5α-androst-3-on-17β-benzoate (dihydrotestosterone benzoate) is described.
65-06-5 Process route
-
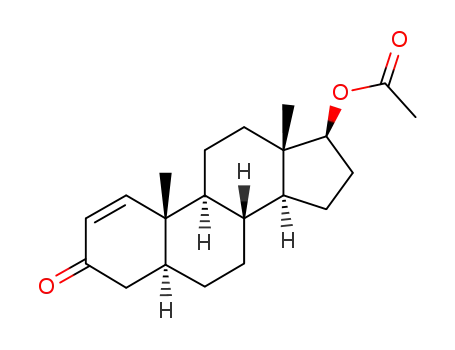
-
64-82-4,6656-41-3,21507-42-6,25615-33-2,54631-35-5,79732-37-9,97413-69-9
(5α,17β)-3-oxoandrost-1-en-17-yl acetate

-
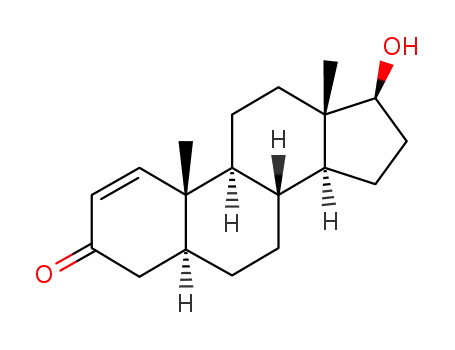
-
65-06-5
1-testosterone
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
methanol;
at 20 ℃;
for 2h;
|
99.8% |
|
With
potassium hydroxide;
In
methanol;
at 20 ℃;
for 1h;
|
-
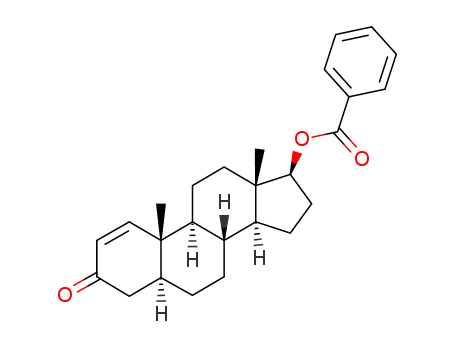
-
1971-67-1
5α-androst-1-en-3-one-17β-yl benzoate

-

-
65-06-5
1-testosterone
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
for 2h;
Heating;
|
82.6% |
65-06-5 Upstream products
-
521-18-6
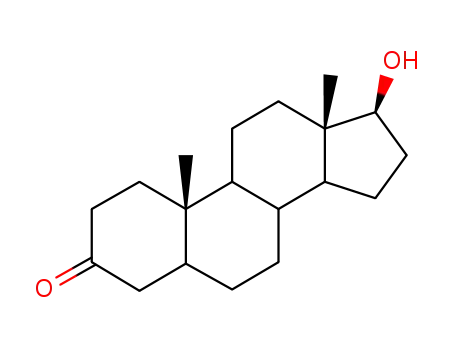
dihydrotestosterone
-
119572-14-4
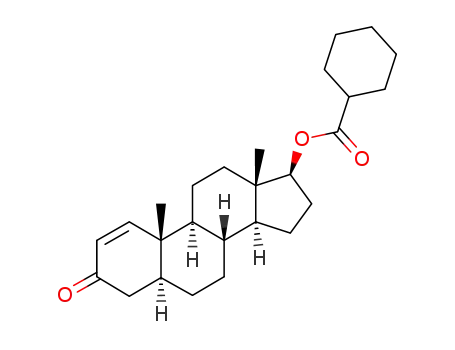
17β-cyclohexanecarbonyloxy-5α-androst-1-en-3-one
-
1971-67-1

5α-androst-1-en-3-one-17β-yl benzoate
-
38859-38-0
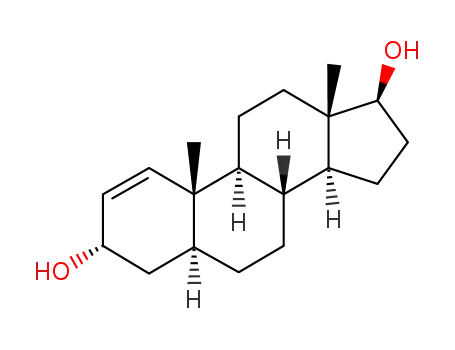
5α-androst-1-ene-3α,17β-diol
65-06-5 Downstream products
-
571-20-0
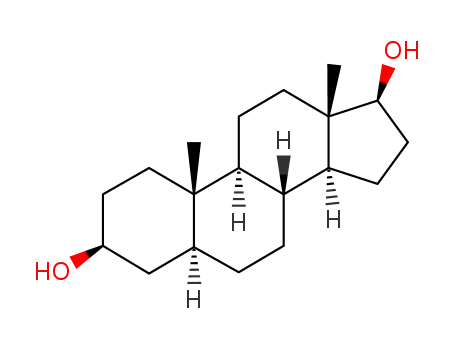
5-androgen-3,17-diol
-
86306-64-1
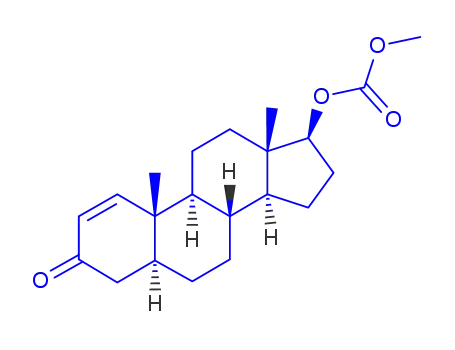
17-β-methoxycarbonyloxy-5α-androst-1-en-3-one
-
7483-09-2
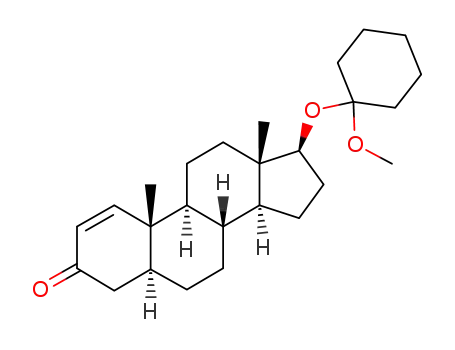
mesabolone
-
1971-67-1

5α-androst-1-en-3-one-17β-yl benzoate








