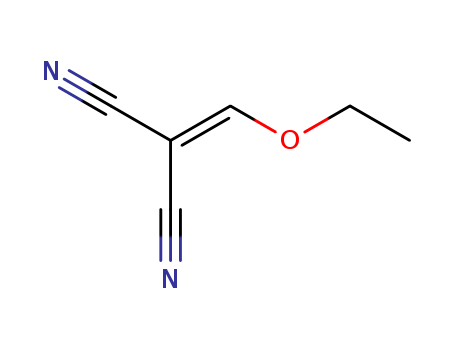
Ethoxymethylenemalononitrile 123-06-8
- CasNo:123-06-8
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
123-06-8 Properties
- Molecular Formula:C6H6N2O
- Molecular Weight:122.126
- Appearance/Colour:off white to pale yellow
- Vapor Pressure:0.00146mmHg at 25°C
- Melting Point:64-66 °C(lit.)
- Refractive Index:1.461
- Boiling Point:296.1 °C at 760 mmHg
- Flash Point:135.1 °C
- PSA:56.81000
- Density:1.07 g/cm3
- LogP:0.95396
123-06-8 Usage
Chemical Properties
Off White to Pale Yellow
Uses
Chemical intermediate.
Uses
Ethoxymethylene Malononitrile (cas# 123-06-8) is a compound useful in organic synthesis.
Synthesis Reference(s)
Tetrahedron Letters, 10, p. 3279, 1969 DOI: 10.1017/S0009838800024678
InChI:InChI=1/C6H6N2O/c1-2-9-5-6(3-7)4-8/h5H,2H2,1H3
123-06-8 Relevant articles
Synthesis and physicochemical properties of merocyanine dyes based on dihydropyridine and fragments of cyanoacetic acid derivatives
Borisova, Irina A.,Zubarev, Andrey A.,Rodinovskaya, Lyudmila A.,Shestopalov, Anatoliy M.
, p. 73 - 86 (2017)
Merocyanine dyes of the dihydropyridine series were prepared from salts of α(γ)-picolinium and cyanoacetic acid derivatives. Their spectral characteristics suggesting their structure in solution were studied. The change in the spectral properties depending on the substituents introduced into the structure of the substituents and solvents used (solvatochromism) was considered. The protonation of the dyes was studied, and its regioselectivity was established.
18F-labeled pyrazolo[1,5-a]pyrimidine derivatives: Synthesis from 2,4-dinitrobenzamide and tosylate precursors and comparative biological evaluation for tumor imaging with positron emission tomography
Xu, Jingli,Liu, Hang,Li, Guixia,He, Yong,Ding, Rui,Wang, Xiao,Feng, Man,Zhang, Shuting,Chen, Yurong,Li, Shilei,Zhao, Mingxia,Li, Yingruo,Qi, Chuanmin,Dang, Yonghong
, p. 3774 - 3793 (2012)
We previously reported 18F-labeled pyrazolo[1,5-a]pyrimidine derivatives: 7-(2-[18F]fluoroethylamino)-5-methylpyrazolo[1,5-a] pyrimidine-3-carbonitrile ([18F]1) and N-(2-(3-cyano-5- methylpyrazolo[1,5-a]pyrimidin-7-ylamino)ethyl)-2-[18F]fluoro-4- nitrobenzamide ([18F]2). Preliminary biodistribution experiments of both compounds showed s slow clearance rate from excretory tissues which warranted further investigation for tumor imaging with PET. Here we modified [18F]1 and [18F]2 by introducing polar groups such as ester, hydroxyl and carboxyl and developed three additional 18F-18 labeled pyrazolo[1,5-a] pyrimidine derivatives: (3-Cyano-7-(2-[ 18F]fluoroethylamino)pyrazolo[1,5-a]-pyrimidin-5- yl)methyl acetate ([18F]3), 7-(2-[18F]fluoroethylamino)-5-(hydroxymethyl) pyrazolo[1,5-a]- pyrimidine-3-carbonitrile ([18F]4) and (S)-6-(3-cyano-5-methylpyrazolo[1,5-a]pyrimidin-7- ylamino)-2-(2-[ 18F]fluoro-4-nitrobenzamido)hexanoic acid ([18F]5). The radiolabeled probes were synthesized by nucleophilic substitution of the corresponding tosylate and nitro precursors with 18F-fluoride. In Vitro studies showed higher uptake of [18F]3 and [18F]4 than that of [18F]5 by S180 tumor cells. In Vivo biodistribution studies in mice bearing S180 tumors showed that the uptake of both [ 18F]3 and [18F]4 in tumors displayed an increasing trend while the uptake of [18F]5 in tumor decreased through the course of the 120 min study. This significant difference in tumor uptake was also found between [18F]1 and [18F]2. Thus, we compared the biological behavior of the five tracers and reported the tumor uptake kinetic differences between 2-[18F]fluoroethylamino- and 2-[ 18F]fluoro-4-nitro- benzamidopyrazolo[1,5-a] pyrimidine derivatives.
Spectroscopic Investigations of Merocyanines-1. UV- and NMR-Spectra of Malodintrile-Substituted Vinylogen Acid Amides.
Scheibe,Schneider,Doerr,Daltrozzo
, p. 630 - 638,631,632,634,636 (1976)
UV and NMR spectra of open chained merocyanine dyes are investigated and model calculations are performed within the Pariser Parr Pople approximation. The introduction of charged pseudo centers permits the calculation of the solvent dependent shifts in the electronic absorption spectra. A good correlation is also found between computed pi -charge densities and observed **1H chemical shifts. Due to the high electronegativity of the malonitrile group, the electronic structure and spectroscopic behavior of the investigated compounds approach that of the symmetrical polymethine dyes.
-
Huber
, (1944)
-
NMR spectroscopic data of some 1-alkoxy-2,2-di(carbonyl, carboxyl, cyano)-substituted ethylenes
Hametner, Christian,Cernuchova, Petra,Milata, Viktor,Vo-Thanh, Giang,Loupy, Andre
, p. 171 - 173 (2005)
The 1H-13C NMR shifts as well as 1H and 13C coupling constants of 14 alkoxymethylene malonic acid and acetoacetic acid derivatives and two alkoxymethylene acetylacetones are reported. The 17O NMR spectra have been recorded for six of them. The long-range coupling 3J(H-C=C-CR) has been used for determining the stereochemistry of the double bond. Copyright
-
Jones
, p. 4889 (1952)
-
-
Kabusz,S.,Tritschler,W.
, p. 312 - 314 (1971)
-
Synthesis and biological evaluation of some acyclic 4,6-disubstituted 1H-pyrazolo[3,4-d]pyrimidine nucleosides
Moukha-Chafiq,Taha,Mouma,Lazrek,Vasseur,De Clercq
, p. 967 - 972 (2003)
The chemical synthesis and biological evaluation of some acyclic α-[6-(1′-carbamoylalkylthio)-1H-pyrazolo[3,4-d]pyrimidin-4-yl] thioalkylamide nucleosides are described.
-
Schmidt,Junek
, p. 895,899 (1977)
-
GLUCOSE UPTAKE INHIBITORS AND USES THEREOF
-
Paragraph 00272; 00420, (2021/05/21)
The present invention relates to novel compounds that modulate cellular glucose uptake by affecting various targets, including, but not limited to those related to glycolysis and known transporters/co-transporters of the GLUT family. The compounds according to the invention are useful for treating cancer such as: neuroendrocrine neoplasms, gastrointestinal stromal tumors (GIST), renal cell carcinoma, paraganglioma, pheochromocytoma, pituitary adenoma, colorectal cancer, lung cancer, gastric cancer, pancreatic cancer sarcoma, head and neck cancer, melanoma, ovarian cancer and other cancers that rely on high levels of glycolysis for survival and proliferation; as well as in treating of autoimmune diseases, inflammation, infectious diseases, and metabolic diseases.
Triflic acid-mediated N-heteroannulation of β-anilino-β-(methylthio)acrylonitriles: a facile synthesis of 4-amino-2-(methylthio)quinolines
Bandyopadhyay, Debashruti,Panigrahi, Adyasha,Peruncheralathan, S.,Radhakrishnan, Divya,Thirupathi, Annaram
supporting information, p. 8544 - 8553 (2021/10/20)
Various functionalised 4-amino-2-(methylthio)quinolines are synthesised through triflic acid-mediated N-heteroannulation of α-functionalized-β-anilino-β-(methylthio)acrylonitriles for the first time. The N-heteroannulation process is highly chemoselective and has mild reaction conditions. However, this process fails in the absence of the β-methylthio group in the acrylonitriles. In addition, a new double N-heteroannulation process is demonstrated to synthesise indolo[3,2-c]quinolines from non-heterocyclic precursors. Natural product isocryptolepine is synthesised in four steps from an acyclic precursor.
Pharmacophore-linked pyrazolo[3,4-d]pyrimidines as EGFR-TK inhibitors: Synthesis, anticancer evaluation, pharmacokinetics, and in silico mechanistic studies
Abulkhair, Hamada S.,Al-Karmalawy, Ahmed A.,Bayoumi, Ashraf H.,El-Adl, Khaled,El-Gamal, Kamal M.,El-Morsy, Ahmed M.,Ezz Eldin, Rogy R.,Gaber, Ahmed A.,Saleh, Marwa A.,Sherbiny, Farag F.
, (2021/09/02)
Targeting the epidermal growth factor receptors (EGFRs) with small inhibitor molecules has been validated as a potential therapeutic strategy in cancer therapy. Pyrazolo[3,4-d]pyrimidine is a versatile scaffold that has been exploited for developing potential anticancer agents. On the basis of fragment-based drug discovery, considering the essential pharmacophoric features of potent EGFR tyrosine kinase (TK) inhibitors, herein, we report the design and synthesis of new hybrid molecules of the pyrazolo[3,4-d]pyrimidine scaffold linked with diverse pharmacophoric fragments with reported anticancer potential. These fragments include hydrazone, indoline-2-one, phthalimide, thiourea, oxadiazole, pyrazole, and dihydropyrazole. The synthesized molecules were evaluated for their anticancer activity against the human breast cancer cell line, MCF-7. The obtained results revealed comparable antitumor activity with that of the reference drugs doxorubicin and toceranib. Docking studies were performed along with EGFR-TK and ADMET profiling studies. The results of the docking studies showed the ability of the designed compounds to interact with key residues of the EGFR-TK through a number of covalent and noncovalent interactions. The obtained activity of compound 25 (IC50 = 2.89 μM) suggested that it may serve as a lead for further optimization and drug development.
Continuous synthesis method of ethoxymethylene malononitrile (by machine translation)
-
Paragraph 0034-0075, (2020/05/08)
The method comprises the following steps, continuously discharging the malononitrile: raw formic acid ester and acetic anhydride into the continuous reaction equipment for replacing the reaction, to obtain the ethoxymethylene malononitrile, and continuously heating the raw materials to the reaction temperature, in a substitution reaction process . and ; The method comprises the following steps of continuously discharging, DEG, malononitrile 1:(0.9-6.0):(2.0-6.0) .raw formic acid ester and acetic anhydride through a continuous reactor, in a continuous reaction device, in a continuous reaction device in a substitution reaction process to obtain the ethyoxyl methylene malononitrile in the continuous reaction equipment at, DEG C in the process of the process of the present invention; and the method comprises the step of continuously, synthesizing the raw materials . The method comprises the following steps of: reducing, safety risks. (by machine translation)
123-06-8 Process route
-
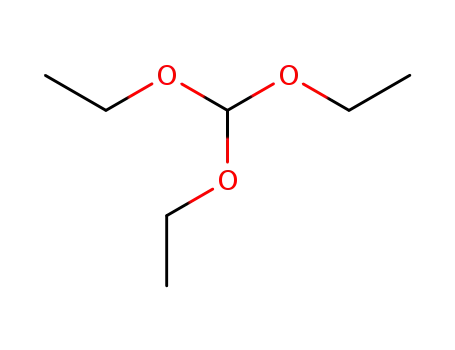
-
122-51-0
orthoformic acid triethyl ester

-
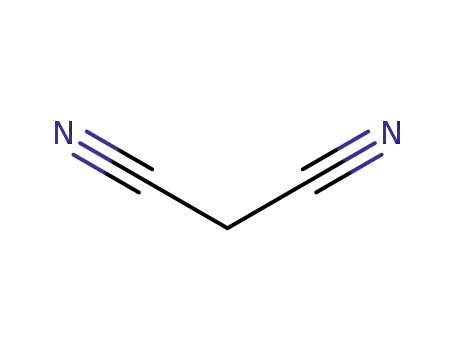
-
109-77-3
malononitrile

-
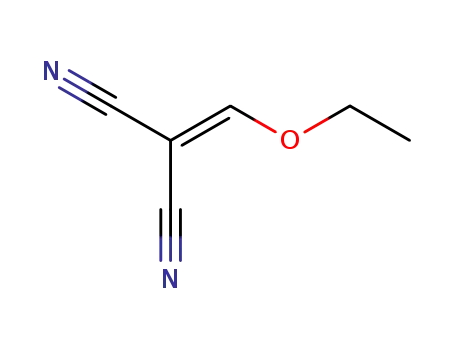
-
123-06-8
Ethoxymethylenemalononitrile
| Conditions | Yield |
|---|---|
|
With
acetic anhydride;
at 110 - 140 ℃;
Reflux;
|
98% |
|
With
acetic anhydride;
at 110 - 140 ℃;
|
98% |
|
With
acetic anhydride;
at 110 - 140 ℃;
|
98% |
|
With
acetic anhydride;
at 110 - 140 ℃;
|
98% |
|
With
acetic anhydride;
at 120 ℃;
under 2250.23 - 3750.38 Torr;
Temperature;
Pressure;
Large scale;
|
98.69% |
|
With
silica gel;
In
N,N-dimethyl acetamide;
for 0.0833333h;
microwave irradiation;
|
95% |
|
In
acetic anhydride;
at 100 ℃;
for 4h;
|
93% |
|
In
acetic anhydride;
for 6h;
Reflux;
|
88% |
|
With
acetic anhydride;
at 0 ℃;
for 18h;
Reflux;
|
84% |
|
With
acetic anhydride;
at 0 ℃;
for 18h;
Reflux;
|
84% |
|
With
acetic anhydride;
for 6h;
Reflux;
|
84.3% |
|
In
acetic anhydride;
Reflux;
|
82% |
|
With
acetic anhydride;
at 100 ℃;
for 4h;
|
78.6% |
|
With
acetic anhydride;
at 100 ℃;
for 4h;
|
78% |
|
With
acetic anhydride;
for 5h;
Reflux;
|
74% |
|
With
acetic anhydride;
|
71% |
|
With
acetic anhydride;
at 90 ℃;
for 12h;
|
65.2% |
|
With
acetic anhydride;
at 140 - 150 ℃;
for 2h;
|
62% |
|
With
acetic anhydride;
at 130 ℃;
for 24h;
Sealed tube;
|
50% |
|
With
acetic anhydride;
at 130 ℃;
for 8h;
|
47.34% |
|
With
acetic anhydride;
|
|
|
With
acetic anhydride;
Heating;
|
|
|
With
acetic anhydride;
KSF Montmorillonite;
at 125 ℃;
for 0.25h;
Further Variations:;
Catalysts;
Product distribution;
microwave irradiation;
|
93 % Chromat. |
|
With
acetic anhydride;
|
|
|
With
acetic anhydride;
at 95 ℃;
for 3h;
|
|
|
With
acetic anhydride;
|
|
|
With
acetic anhydride;
at 140 ℃;
for 36h;
|
|
|
With
acetic anhydride;
at 130 ℃;
for 5h;
|
|
|
With
acetic anhydride;
at 140 ℃;
for 6h;
|
|
|
With
acetic anhydride;
for 5h;
Reflux;
|
|
|
With
acetic anhydride;
In
ethanol;
at 100 ℃;
|
-

-
109-77-3
malononitrile

-

-
123-06-8
Ethoxymethylenemalononitrile
| Conditions | Yield |
|---|---|
|
With
orthoformic acid triethyl ester;
at 140 ℃;
|
|
|
With
orthoformic acid triethyl ester;
at 140 ℃;
|
123-06-8 Upstream products
-
108-24-7
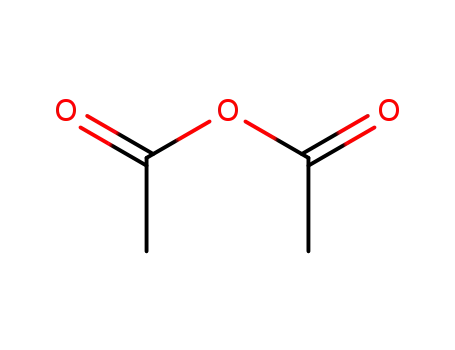
acetic anhydride
-
122-51-0

orthoformic acid triethyl ester
-
109-77-3

malononitrile
-
79343-59-2
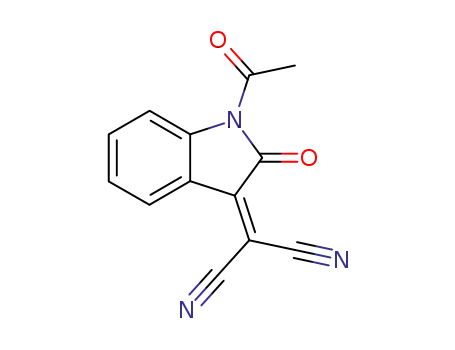
1-acetyl-3-(dicyanomethylene)-1,3-dihydro-2H-indol-2-one
123-06-8 Downstream products
-
21254-23-9
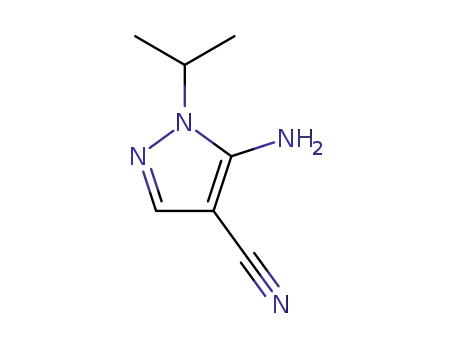
5-Amino-4-cyan-1-isopropyl-pyrazol
-
16462-27-4
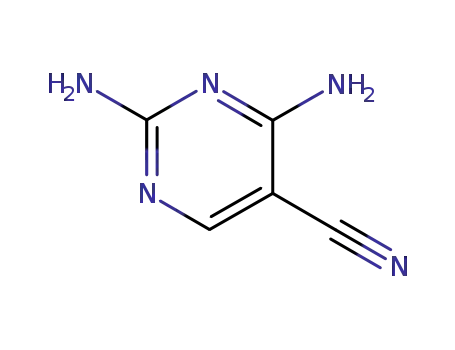
2,4-diamino-pyrimidine-5-carbonitrile
-
698-29-3
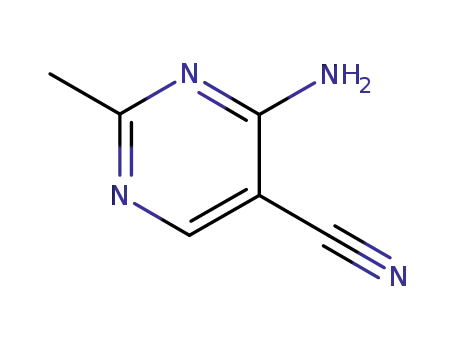
2-methyl-4-amino-5-cyanopyrimidine
-
76574-45-3
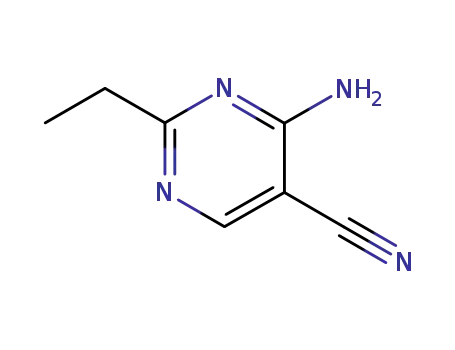
4-amino-2-ethylpyrimidine-5-carbonitrile








