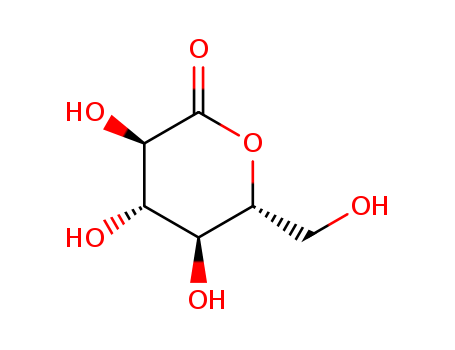
D-(+)-Glucono-1,5-lactone 90-80-2
- CasNo:90-80-2
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
90-80-2 Properties
- Molecular Formula:C6H10O6
- Molecular Weight:178.142
- Appearance/Colour:Powder
- Vapor Pressure:7.6E-10mmHg at 25°C
- Melting Point:151-155 °C
- Refractive Index:63.5 ° (C=10, H2O)
- Boiling Point:446.417 °C at 760 mmHg
- PKA:12.06±0.60(Predicted)
- Flash Point:192.294 °C
- PSA:107.22000
- Density:1.721 g/cm3
- LogP:-3.01320
90-80-2 Usage
Description
Delta-Gluconolactone (GDL) is a lactone of the D-gluconate. It is a natural constituent of many foods. It can be found in honey, fruit juices, wine and many-fermented products1-3. It is used as a food additive with the E number E575 used as a sequestrant, an acidifier (it lower the pH and also help preserve the food from deterioration by enzymes and organisms), or a curing, pickling, or leavening agent. GDL has been marketed for use in feta cheese. GDL is neutral, but hydrolyses in water to gluconic acid that is acidic, adding a tangy taste to foods, though it has roughly a third of the sourness of citric acid. It can be used as nutritional supplement in beverage such as in Instant Drinks, Syrups, RTD Tea and Coffee, Sports and Energy Drinks, Waters.
Description
Glucono delta-lactone (C6H10O6), molecular weight 178.14, is an inner ester of gluconic acid. Commonly named gluconolactone, other synonyms include D-gluconic acid delta-lactone, D-glucono- 1, 5-lactone, and D-delta-gluconolactone. Some of its earliest uses as a food ingredient were as a flavoring (e.g., sherbets) and to reduce fat absorption in doughnuts and cones. Glucono deltalactone tastes sweet initially and has a slightly acid-aftertaste.
Chemical Properties
Powder
Uses
Geogard(R)Ultra is a synergistic blend of gluconolactone and sodium benzoate. This blend provides broad spectrum protection against product spoilage in a variety of personal care formulations. Product Data Sheet
Uses
A lactone (cyclic ester) of D-gluconic acid used as a used as a sequestrant, an acidifier, or a curing, pickling, or leavening agent.
Uses
D-glucono-1,5-lactone is the lactone derivative of D-gluconic acid. D-glucono-1,5-lactone is widely used as a food additive to achieve a curing, pickling or leavening effect.
Uses
a product of the oxidation of glucose by glucose oxidase
Uses
Component of many cleaning cmpds because of the sequestering ability of the gluconate radical which remains active in alk solutions; in the dairy industry to prevent milkstone; in breweries to prevent beerstone; as latent acid catalyst for acid colloid resins, particularly in textile printing; as a coagulant for tofu.
Uses
gluconolactone is used in cosmetics for its anti-acne properties. It can also help improve skin hydration given its water-binding ability. In addition, formulators may select gluconolactone for its action as a product stabilizer (chelating agent). Some studies indicate potential free-radical scavenging capacities as well. These properties would make it particularly relevant for use in making anti-aging, moisturizing, and possibly sun care products.
Uses
(GDL) An acidulant. It hydrolyzes to form gluconic acid in water solution and thereby creates the desired pH. The rate of acid formation is affected by temperature, concentration, and the pH of the solution. It has low acid release at room temperature and accelerated conversion into gluconic acid at high temperatures. It is readily soluble with a solubility of 59 g in 100 ml of water at 20°C. It functions as a leavening agent, acidulant, curing and pickling agent, and pH control agent. It is comparatively less tart/sour than other food acids. It is used in baked goods, fish products, desserts, and dressings.
Preparation
Glucono delta-lactone is prepared commercially by the oxidation of glucose with bromine water.
General Description
Gluconolactone is a non-toxic component of the skin. It has anti-oxidant and free radical scavenging effects. Gluconolactone has antiaging and skin-firming properties. It acts as a?β-glucosidase inhibitor. Gluconolactone stimulates cellulase gene expression.
Flammability and Explosibility
Nonflammable
Biochem/physiol Actions
Glucono-d-lactone increased the doubling time and activated enzymes involved in the oxidative pentose phosphate pathway of Saccharomyces cerevisiae.
Safety Profile
Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise Dglucono-lactone from ethylene glycol monomethyl ether and dry for 1hour at 110o. It can be freed from other sugars via a column of Celite and charcoal (750g of each, 90 x 7.5cm) which is washed with 0.01N formic acid until the pH of the wash is equal to that of the entering acid. The lactone is applied in H2O and eluted with 0.01N formic acid (7L), then eluted with 7.5% EtOH/0.01N formic acid (8L), then 15% EtOH/0.01N formic acid (8L) which removes pentose and isomaltose (the optical rotation of the eluates are used for sugar detection) and finally elution with aqueous formic acid provides glucolactone which is obtained by evaporating or freeze drying. Its solubility in H2O is 60% and 1% in EtOH. A solution in H2O is slightly acidic, and the lactone dissolves in an equivalent of aqueous NaOH to form sodium D-gluconate [527-07-1] M 218.1, m 2002 0 6o(dec), [ ] D 25 +12o (c 10, H2O), pK2 5 3.6. [cf p 553, Smith & Whelan Biochemical Preparations 10 127 1963, Beilstein 3 IV 1255.]
InChI:InChI=1/C6H10O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-5,7-10H,1H2/t2-,3-,4+,5-/m0/s1
90-80-2 Relevant articles
Efficient improvement in non-enzymatic glucose detection induced by the hollow prism-like NiCo2S4electrocatalyst
Chen, Qiwen,Chen, Xiaojun,Chu, Dandan,Chu, Xue-Qiang,Ge, Danhua,Yan, Li
, p. 15162 - 15169 (2021/11/17)
Hollow prism-like NiCo2S4 materials (NiCo2S4 HNPs) were successfully fabricated by a two-step method. Scanning electron microscopy (SEM), transmission electron microscopy (TEM) and powder X-ray diffraction (XRD) confirmed the morphology and structure of the as-prepared NiCo2S4 nanoprisms. A non-enzymatic sensor based on NiCo2S4 HNPs was constructed with outstanding electrochemical activity towards glucose oxidation in alkaline medium. The sensor showed a rapid response time (~0.1 s), a high sensitivity of 82.9 μA mM-1 cm-2, a wide linear range (0.005-20.2 mM) and a detection limit of 0.8 μM (S/N = 3) with a good selectivity and reproducibility. Additionally, the proposed electrode also confirmed the feasibility in practical blood serum. These results indicate that NiCo2S4/ITO has great potential in the development of non-enzymatic glucose sensor applications.
carba Nicotinamide Adenine Dinucleotide Phosphate: Robust Cofactor for Redox Biocatalysis
D?ring, Manuel,Sieber, Volker,Simon, Robert C.,Tafertshofer, Georg,Zachos, Ioannis
supporting information, p. 14701 - 14706 (2021/05/13)
Here we report a new robust nicotinamide dinucleotide phosphate cofactor analog (carba-NADP+) and its acceptance by many enzymes in the class of oxidoreductases. Replacing one ribose oxygen with a methylene group of the natural NADP+ was found to enhance stability dramatically. Decomposition experiments at moderate and high temperatures with the cofactors showed a drastic increase in half-life time at elevated temperatures since it significantly disfavors hydrolysis of the pyridinium-N?glycoside bond. Overall, more than 27 different oxidoreductases were successfully tested, and a thorough analytical characterization and comparison is given. The cofactor carba-NADP+ opens up the field of redox-biocatalysis under harsh conditions.
Anticancer and antileishmanial in vitro activity of gold(I) complexes with 1,3,4-oxadiazole-2(3H)-thione ligands derived from δ-D-gluconolactone
Espinosa, Andrés Villase?or,Costa, Danilo de Souza,Tunes, Luiza Guimar?es,Monte-Neto, Rubens L. do,Grazul, Richard Michael,de Almeida, Mauro Vieira,Silva, Heveline
, p. 41 - 50 (2020/07/28)
Four gold(I) complexes conceived as anticancer agents were synthesized by reacting [Au(PEt3)Cl] and [Au(PPh3)Cl] with ligands derived from δ-d-gluconolactone. The ligands’ structure was designed to combine desired biological properti
The non-enzymatic electrochemical detection of glucose and ammonia using ternary biopolymer based-nanocomposites
Bano, Sayfa,Ganie, Adil Shafi,Sultana, Saima,Khan, Mohammad Zain,Sabir, Suhail
, p. 8008 - 8021 (2021/05/21)
The non-enzymatic electrochemical detection of glucose and aqueous ammonia was carried out using ternary bio-nanocomposites. A co-precipitation method was employed to synthesize cadmium stannate nanoparticles, while bio-nanocomposites of polyaniline-cadmium stannate-chitosan were synthesized via the chemical oxidative polymerization technique. Functional group analysis, structural and morphological studies were further carried out via Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and scanning and transmission electron microscopy techniques. The electrode kinetics and electrochemical properties were validated via cyclic voltammetry, electrochemical impedance spectroscopy (EIS), and chronoamperometric techniques. The sensing properties of the electrocatalysts were explored using cyclic voltammetry and amperometry for the detection of glucose and ammonia solution of varying concentrations using NaOH electrolyte. The electrochemical activity of the electrocatalysts were optimized using different weight percentages of chitosan, and bio-nanocomposites of polyaniline/cadmium stannate/chitosan (10%) showed excellent electrocatalytic and electrochemical activities. Moreover, the bio-nanocomposites have a low detection limit and short response time toward different concentrations of glucose and ammonia solution. Apart from these studies, the sensors have also been evaluated for an anti-interference study. The developed novel sensors present significant potential to be efficiently utilized on a large scale, as they also show effective stability and reproducibility, along with a high level of sensitivity for the given target analytes. This journal is
90-80-2 Process route
-
-
740753-96-2
L-allonic acid

-
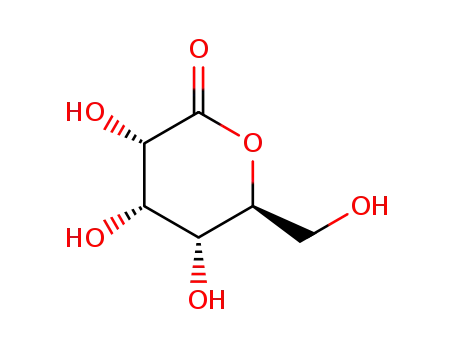
-
90-80-2,7506-65-2,10366-75-3,15892-28-1,32746-79-5,52153-09-0,52393-78-9,110115-45-2,120523-34-4,124819-01-8,124915-65-7,1335-57-5,4253-68-3
L-allonic acid-5-lactone
| Conditions | Yield |
|---|---|
|
With
ethanol;
schnelles Eindampfen unter vermindertem Druck und Behandeln des Rueckstands mit wasserfreiem Aethanol;
|
-
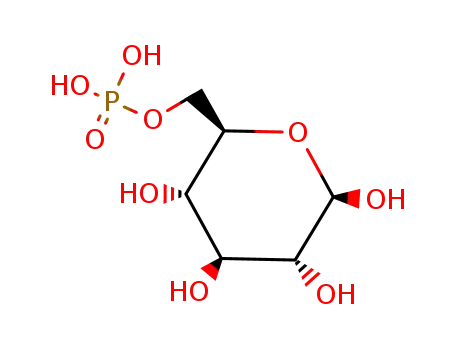
-
299-31-0,15209-11-7,15209-12-8,33163-99-4,40436-57-5,40436-58-6,40436-60-0,40436-61-1
β-D-glucose 6-phosphate

-
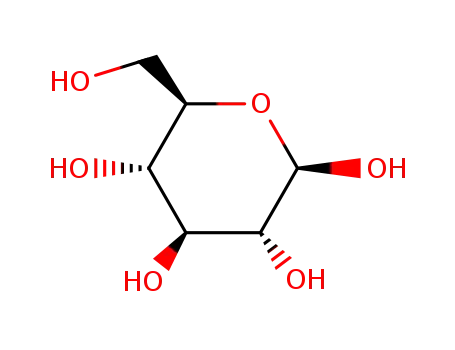
-
492-61-5
β-D-glucose

-
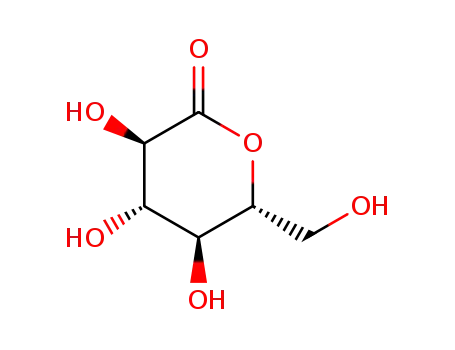
-
90-80-2,1335-57-5,4253-68-3
D-Glucono-1,5-lactone
| Conditions | Yield |
|---|---|
|
With
Yersinia mollaretii phytase; water; oxygen; glucose oxidase;
In
aq. phosphate buffer;
Combinatorial reaction / High throughput screening (HTS);
Enzymatic reaction;
|
90-80-2 Upstream products
-
526-95-4
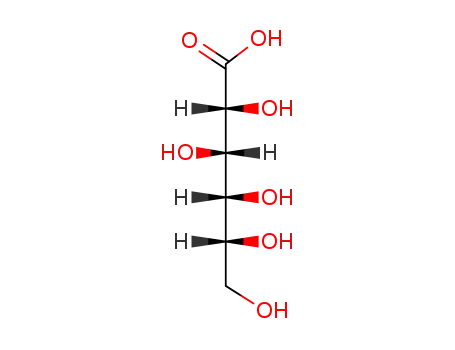
gluconic acid
-
74421-63-9
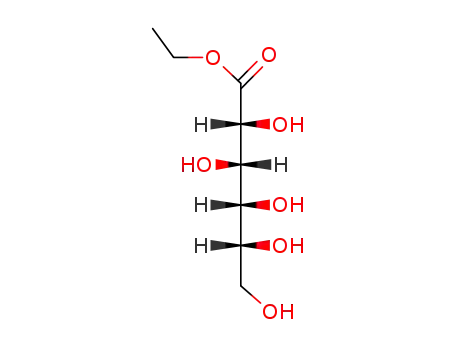
ethyl D-mannonate
-
2280-44-6
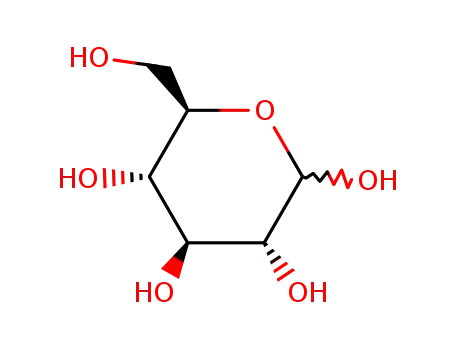
D-Glucose
-
865-05-4
nicotinamide adenine dinucleotide
90-80-2 Downstream products
-
5323-77-3
2,4:3,5-dimethylene-D-gluconic acid
-
13149-69-4
O6-(bis-diisopropylamino-acetyl)-D-gluconic acid
-
28567-53-5
N-((Ξ)-2-ethyl-hexyl)-D-gluconamide
-
18375-63-8
2,3,4,5,6-pentahydroxy hexanoic acid dodecylamide








