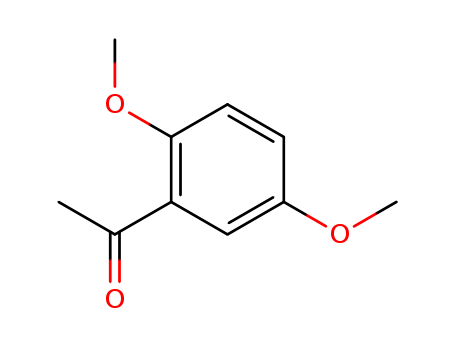
2,5-Dimethoxyacetophenone 1201-38-3
- CasNo:1201-38-3
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
1201-38-3 Properties
- Molecular Formula:C10H12O3
- Molecular Weight:180.203
- Appearance/Colour:clear yellow to green-yellow liquid
- Melting Point:18-20 ºC
- Refractive Index:n20/D 1.5441(lit.)
- Boiling Point:294.3 °C at 760 mmHg
- Flash Point:111.3 °C
- PSA:35.53000
- Density:1.139 g/cm3
- LogP:1.90640
1201-38-3 Usage
Chemical Properties
clear yellow to green-yellow liquid
Uses
2'',5''-Dimethoxyacetophenone is a useful building block which has been used in the preparation of pyridine and thieno[2,3-b] pyridine derivatives as anticancer PIM-1 kinase inhibitors, and in the synthesis of anti-cancer and apoptosis-inducing agents.
Preparation
Obtained by reaction of dimethyl sulfate with quinacetophenone in the presence of 10% aqueous sodium hydroxide.
1201-38-3 Relevant articles
Solid supported Ru(0) nanoparticles: An efficient ligand-free heterogeneous catalyst for aerobic oxidation of benzylic and allylic alcohol to carbonyl
Das, Pralay,Aggarwal, Nidhi,Guha, Nitul Ranjan
, p. 2924 - 2928 (2013)
Polymer immobilized stable, spherical ruthenium nanoparticles were prepared, characterized, and acted as a heterogeneous catalyst for the selective benzylic and allylic alcohol oxidation into the corresponding carbonyls using molecular oxygen. The solid supported Ru(0) (SS-Ru) as a heterogeneous catalyst exhibits good reusability and easy separation from the reaction mixture by filtration.
Design, synthesis and docking study of pyridine and thieno[2,3-b] pyridine derivatives as anticancer PIM-1 kinase inhibitors
Abdelaziz, Marwa E.,El-Miligy, Mostafa M.M.,Fahmy, Salwa M.,Mahran, Mona A.,Hazzaa, Aly A.
, p. 674 - 692 (2018)
A series of pyridine and thieno[2,3-b]pyridine derivatives have been designed and synthesized as anticancer PIM-1 kinase inhibitors. Thirty-seven compounds were selected by NCI to be tested initially at a single dose (10 μM) in the full NCI 60 cell line panel. Compound 5b showed potent anticancer activity and was tested twice in the five-dose assay which confirmed its potent antitumor activity (GI50 values 0.302–3.57 μM) against all tested tumor cell lines except six cell lines where they showed moderate sensitivity. This compound was sent to NCI biological evaluation committee and still under consideration for further testing. In addition, the most active anticancer compounds in each series, 5b, 8d, 10c, 13h, and 15e, were evaluated for their PIM-1 kinase inhibitory activity. Compound 8d was the most potent one with IC50 = 0.019 μM followed by 5b, 15e, 10c and 13h with IC50 values 0.044, 0.083, 0.128 and 0.479 μM respectively. Moreover, docking study of the most active compounds in PIM-1 kinase active site was consistent with the in vitro activity.
Solid-supported Pd(0): An efficient heterogeneous catalyst for aerobic oxidation of benzyl alcohols into aldehydes and ketones
Bandna,Aggarwal, Nidhi,Das, Pralay
, p. 4954 - 4956 (2011)
Solid-supported nano- and microparticles of Pd(0) (SS-Pd) were used as heterogeneous catalysts for aerobic oxidation of benzyl alcohols. Primary and secondary benzyl alcohols gave the corresponding products in good yields. In addition, the catalysts could be reused up to five runs without significant loss of activities.
Phytochemistry of Genus Gentiana. XXVI. Identification of a New Di-O-glucosyl Cinnamoyl-C-glucosylflavone in the Leaves of Gentiana X marcailhouana RY.
Luong, Minh Duc,Saeby, Jan,Fombasso, Pierre,Jacot-Guillarmod, Andre
, p. 2741 - 2745 (1981)
A new di-O-glucosyl cinnamoyl-C-glucosylflavone has been identified as 4'-O-β-D-glucosyl-2''-O-isoorientin (1).
-
Dhami,K.S.,Stothers,J.B.
, p. 479 (1965)
-
Preparation method of 2, 5-dimethoxyphenylacetic acid
-
Paragraph 0063-0066, (2021/01/29)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2, 5-dimethoxyphenylacetic acid, which comprises the following steps: A. Reacting 1,4-dimethoxybenzene with formaldehyde under the action of hydrogen halide, extracting by the reaction system, merging with organic phases, drying, concentrating under reduced pressure, and purifying the crude product to obtain 2-halon methyl-1, 4-dimethoxybenzene ; and B, under the protection of inert gas, putting the 2-halo methyl-1, 4-dimethoxybenzene obtained in the step A, magnesium or butyl lithium and carbon dioxide into a solvent, and then carrying out extraction and deactivation, reduced pressure distillation, extraction, organic phase combination and reduced pressure concentration toobtain the 2, 5-dimethoxyphenylacetic acid. The yield and the total yield of the 2, 5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of the 2,5-dimethoxyphenylacetic acid synthesized by a Willgerodt-Kindler method.
Preparation method of 2,5-dimethoxyphenylacetic acid
-
Paragraph 0071; 0072, (2021/02/06)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2,5-dimethoxyphenylacetic acid, wherein the preparation method comprises the following steps: A, reacting 1,4-dimethoxybenzene with formaldehyde under the action of hydrogen halide to obtain 2-halomethyl-1,4-dimethoxybenzene; B, reacting 2-halomethyl-1,4-dimethoxybenzene obtained inthe step A with a cyanation reagent, then diluting, washing, drying and concentrating under reduced pressure, and finally carrying out reduced pressure distillation to obtain 2(2,5-dimethoxyphenyl)acetonitrile; and C, hydrolyzing the 2(2,5-dimethoxyphenyl)acetonitrile obtained in the step B, cooling, extracting, merging, drying, concentrating under reduced pressure and purifying to finally obtainthe 2,5-dimethoxyphenylacetic acid. The yield and the total yield of the 2,5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of the 2,5-dimethoxyphenylacetic acid synthesized by a Willgerodt-Kindler method, and the yield and the total yield of the 2,5-dimethoxyphenylacetic acid are higher than those of 2,5-dimethoxyphenylacetic acid synthesized by the Willgerodt-Kindler method.
Preparation method of 2,5-dimethoxyphenylacetic acid
-
Paragraph 0096-0097, (2021/02/06)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2,5-dimethoxyphenylacetic acid, wherein the method comprises the following steps: A,reacting 1,4-dimethoxybenzene in a formylation system to obtain 2,5-dimethoxybenzaldehyde; B, reacting the 2,5-dimethoxybenzaldehyde obtained in the step A with a reducing agent, extracting a reaction system, then combining organic phases, drying, concentrating under reduced pressure and distilling a crude product to obtain 2,5-dimethoxybenzyl alcohol; C, reacting the 2,5-dimethoxybenzyl alcoholobtained in the step B with a bromination reagent to obtain 2-bromomethyl-1,4-dimethoxybenzene; and D, reacting the 2-bromomethyl-1,4-dimethoxybenzene obtained in the step C with magnesium or butyl lithium and carbon dioxide in a solvent to obtain the 2,5-dimethoxyphenylacetic acid. The yield and the total yield of the 2,5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of 2,5-dimethoxyphenylacetic acid synthesized by a Willegerdt-Kindler method.
Imidazolium Triflate Ionic Liquid Improves the Activity of ZnCl2 in the Synthesis of Pyrroles and Ketones
Nguyen, Hai Truong,Ngo, Dung Kim Thi,Chau, Khiem Duy Nguyen,Tran, Phuong Hoang
, p. 157 - 165 (2021/03/16)
-
1201-38-3 Process route
-
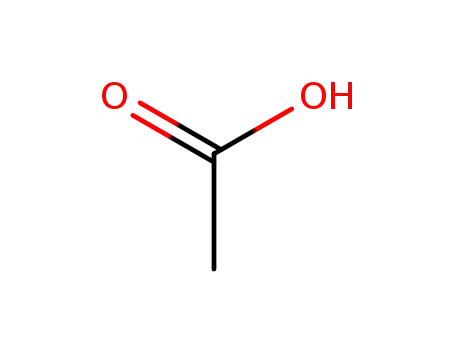
-
64-19-7,77671-22-8
acetic acid

-
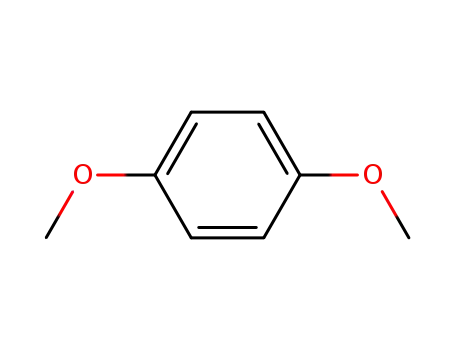
-
150-78-7
1,4-dimethoxybezene

-
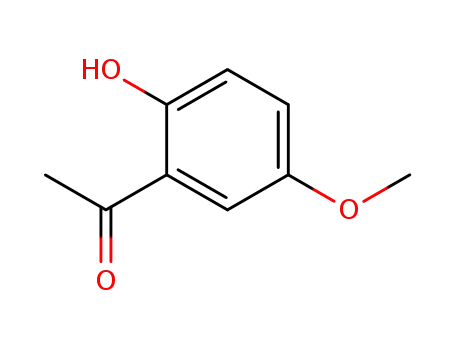
-
705-15-7
1-(2-hydroxy-5-methoxyphenyl)ethan-1-one

-
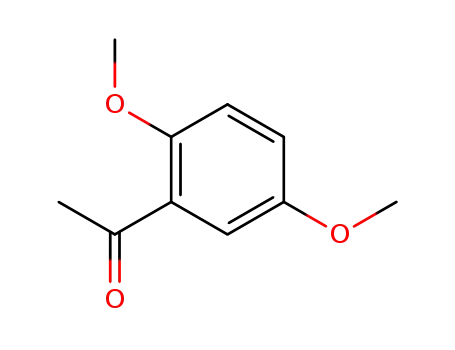
-
1201-38-3
2',5'-dimethoxyacetophenone
| Conditions | Yield |
|---|---|
|
With
boron trifluoride;
|
-
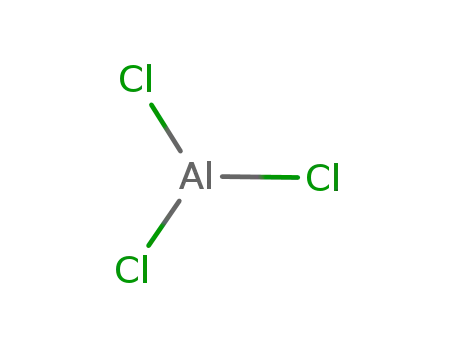
-
7446-70-0
aluminium trichloride

-
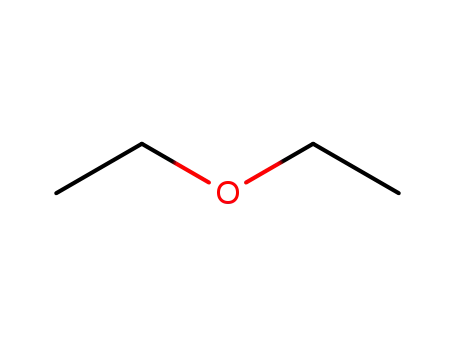
-
60-29-7,927820-24-4
diethyl ether

-
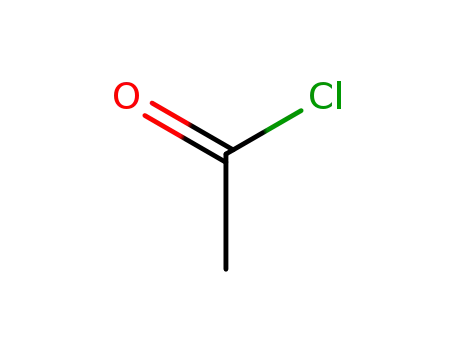
-
75-36-5
acetyl chloride

-

-
150-78-7
1,4-dimethoxybezene

-

-
705-15-7
1-(2-hydroxy-5-methoxyphenyl)ethan-1-one

-

-
1201-38-3
2',5'-dimethoxyacetophenone
| Conditions | Yield |
|---|---|
|
|
1201-38-3 Upstream products
-
108-24-7
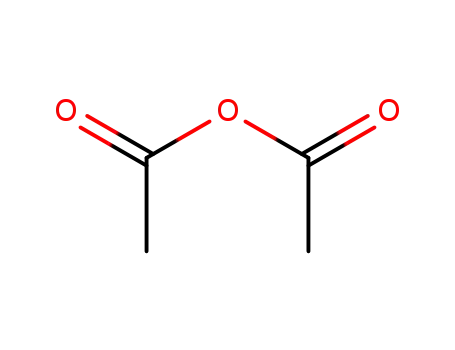
acetic anhydride
-
150-78-7

1,4-dimethoxybezene
-
64-19-7

acetic acid
-
75-36-5

acetyl chloride
1201-38-3 Downstream products
-
145091-58-3
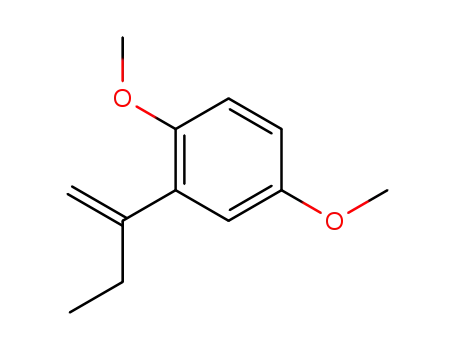
2-(1-ethyl-vinyl)-1,4-dimethoxy-benzene
-
72667-90-4
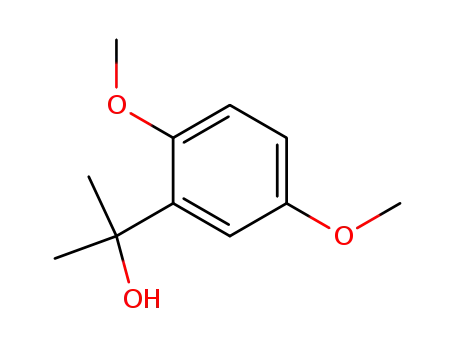
dimethyl-1,4-dimethoxyphenylcarbinol
-
485335-52-2
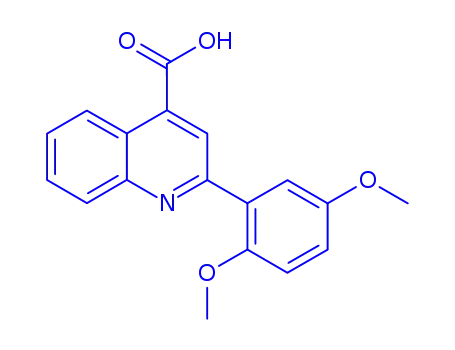
2-(2,5-dimethoxy-phenyl)-quinoline-4-carboxylic acid
-
109469-64-9
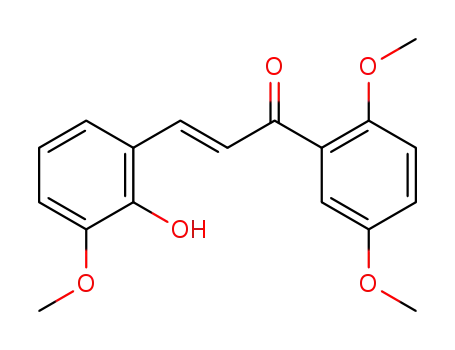
2-hydroxy-3,2',5'-trimethoxy-trans-chalcone








