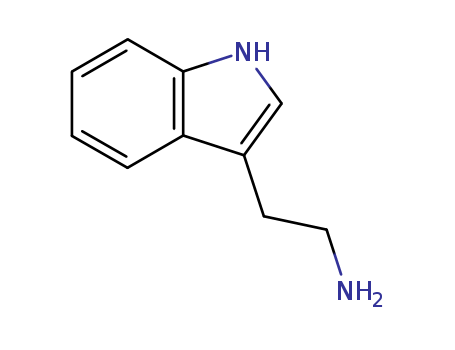
Product Details
61-54-1 Properties
- Molecular Formula:C10H12N2
- Molecular Weight:160.219
- Appearance/Colour:White to orange crystalline powder
- Vapor Pressure:6.14E-06mmHg at 25°C
- Melting Point:113-116 °C(lit.)
- Refractive Index:1.668
- Boiling Point:378.766 °C at 760 mmHg
- PKA:10.2(at 25℃)
- Flash Point:187.672 °C
- PSA:41.81000
- Density:1.158 g/cm3
- LogP:2.36940
61-54-1 Usage
Description
Tryptamine is a monoamine alkaloid that can be synthesized by decarboxylation of the amino acid tryptophan. Notably, tryptamine can be found in fungi, plants, Amphibia, animals, and microbes. Tryptamine has an indole ring structure and a fused double ring that is composed of a benzene ring and a pyrrole ring, linked to an amino group by 2-carbon side chain. The indole ring is the vital nucleus of many complex natural products that have significance in drug discovery as well as some synthetic and non-synthetic drugs that are based on tryptamine skeleton. The chemical’s distinct structure is an approximation to the neurotransmitter serotonin as well-known drugs and hallucinogens. Tryptamine’s significance as psychedelic drugs, neuromodulator, and neurotransmitter is well understood due to its presence in mammalian brain in small amounts.
Applications
Analogs of tryptamine that are typically produced by its synthetic modification play a significant role in individuals due to the introduction of functionalities that are biologically active in its nucleus that may cause changes in the mental and physical status of the human brain. Substitutions on the indole ring at nitrogen and C-2 of its side chain produce numerous neuroactive compounds ranging from anti-migraine drugs to toxic substances, such as rizatriptan, sumatriptan, and zolmitriptan. A small amount of tryptamine is required due to its fatalities and intoxication for several reasons.
Plants Containing Tryptamine
In plants, tryptamine in small amounts acts as a promising phase to the plant hormone indole-3-acetic acid in one biosynthetic pathway. N, N-dimethyltryptamine (DMT) is a tryptamine derivative that is an active constituent for the hallucinogenic effect of brew known as the “vine of the souls.” Indigenous Amazonian tribes have traditionally used the drink for therapeutic purposes for effective treatment of some physical maladies and abuse disorders. Magic mushrooms are the most common fungi that contain tryptamine derivatives.
Pharmacology
Serotonin (5-hydroxytryptamine, 5-HT), which is a natural derivative of tryptamine is a significant signaling hormone that helps in the modulation and regulation of numerous processes within the central nervous system, for instance, cognition, sleep, temperature regulation, memory, and behavior. The mammalian brain contains small traces of tryptamine, which generally act as a modulator or neurotransmitter by releasing serotonin agents. It is also an enhancer of serotonergic activity. Only one minute is required for tryptamine to produce psychotropic phenomena when used recreationally. It has been associated with fatalities and intoxications for multiple reasons including low toxic concentrations.
Description
Tryptamine is an indole alkaloid and intermediate in the biosynthesis of serotonin and the phytohormone melatonin in plants. It increases the levels of the terpenoid indole alkaloids ajmalicine, strictosidine, and catharanthine in cultures of C. roseus. Tryptamine is also a product of tryptophan metabolism in mammals. Tryptamine derivatives have been synthetically produced as hallucinogenic drugs of abuse that act on the serotonergic system.
Chemical Properties
Tryptamine is a biogenic imine derived from the decarboxylation of tryptophan. It is a white to orange crystalline Powder, melting point 118°C (decomposition at 145-146°C). Soluble in ethanol and acetone, almost insoluble in ether, benzene, chloroform and water.
Uses
Tryptamine is a monoamine alkaloid found in plants. Tryptamine is commonly used in the preparation of biologically active compounds such as neurotransmitters and psychedelics.
Definition
ChEBI: Tryptamine is an aminoalkylindole consisting of indole having a 2-aminoethyl group at the 3-position. It has a role as a human metabolite, a plant metabolite and a mouse metabolite. It is an aminoalkylindole, an indole alkaloid, an aralkylamino compound and a member of tryptamines. It is a conjugate base of a tryptaminium.
Preparation
Tryptamine, a monoamine alkaloid containing an indole ring structure is derived by the decarboxylation of amino acid tryptophan.The synthesis of tryptamines is typically conducted following a classic route starting with a Mannich reaction of an indole heterocycle, followed by quaternization of the amine, nucleophilic substitution with highly toxic cyanide and final reduction.
General Description
Tryptamines which are usually found in plants, fungi, animals, etc. are categorized under the monoamine alkaloids class of compounds.
Biochem/physiol Actions
Vasoactive; may have a neuromodulator function; biogenic amine formed from the decarboxylation of tryptophan by L-aromatic amino acid decarboxylase.
Purification Methods
Crystallise tryptamine from *benzene, Et2O (m 114o) or pet ether (m 118o). It has UV: 222n 276, 282 and 291nm (EtOH) and max 226, 275, 281 and 290nm (HCl). [Beilstein 22 II 346, 22 III/IV 4319, 22/10 V 45.]
InChI:InChI=1/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2
61-54-1 Relevant articles
-
Brutcher,Vanderwerff
, p. 146 (1958)
-
Concerning the preparation of 6-bromotryptamine
Scott Wiens,Johnson, Jerry L.,Gribble, Gordon W.
, (2021/03/15)
Most of the previous syntheses of the marine natural product 6-bromotryptamine have almost certainly led to partial debromination resulting in an impure product containing tryptamine. We show that loss of bromine occurs when lithium aluminum hydride is employed as a reducing agent in the final reaction step leading to 6-bromotryptamine. Reductive-debromination is also likely to intrude during some of the syntheses of 6-bromoindole, the typical precursor to 6-bromotryptamine. None of the seven described syntheses of 6-bromotryptamine that involve a reduction sequence from 6-bromoindole have reported elemental analyses as a measure of purity.
1-BENZYLSPIRO[PIPERIDINE-4,1′-PYRIDO[3,4-b]indole] ‘co-potentiators’ for minimal function CFTR mutants
Son, Jung-Ho,Phuan, Puay-Wah,Zhu, Jie S.,Lipman, Elena,Cheung, Amy,Tsui, Ka Yi,Tantillo, Dean J.,Verkman, Alan S.,Haggie, Peter M.,Kurth, Mark J.
, (2020/10/26)
We previously identified a spiro [piperidine-4,1-pyrido [3,4-b]indole] class of co-potentiators that function in synergy with existing CFTR potentiators such as VX-770 or GLGP1837 to restore channel activity of a defined subset of minimal function cystic fibrosis transmembrane conductance regulator (CFTR) mutants. Here, structure-activity studies were conducted to improve their potency over the previously identified compound, 20 (originally termed CP-A01). Targeted synthesis of 37 spiro [piperidine-4,1-pyrido [3,4-b]indoles] was generally accomplished using versatile two or three step reaction protocols with each step having high efficiency. Structure-activity relationship studies established that analog 2i, with 6′-methoxyindole and 2,4,5-trifluorobenzyl substituents, had the greatest potency for activation of N1303K-CFTR, with EC50 ~600 nM representing an ~17-fold improvement over the original compound identified in a small molecule screen.
Multitarget Biological Profiling of New Naphthoquinone and Anthraquinone-Based Derivatives for the Treatment of Alzheimer's Disease
Campora, Marta,Canale, Claudio,Gatta, Elena,Tasso, Bruno,Laurini, Erik,Relini, Annalisa,Pricl, Sabrina,Catto, Marco,Tonelli, Michele
, p. 447 - 461 (2021/02/01)
Two series of naphthoquinone and anthraquinone derivatives decorated with an aromatic/heteroaromatic chain have been synthesized and evaluated as potential promiscuous agents capable of targeting different factors playing a key role in Alzheimer's disease (AD) pathogenesis. On the basis of the in vitro biological profiling, most of them exhibited a significant ability to inhibit amyloid aggregation, PHF6 tau sequence aggregation, acetylcholinesterase (AChE), and monoamine oxidase (MAO) B. In particular, naphthoquinone 2 resulted as one of the best performing multitarget-directed ligand (MTDL) experiencing a high potency profile in inhibiting β-amyloid (Aβ40) aggregation (IC50 = 3.2 μM), PHF6 tau fragment (91% at 10 μM), AChE enzyme (IC50 = 9.2 μM) jointly with a remarkable inhibitory activity against MAO B (IC50 = 7.7 nM). Molecular modeling studies explained the structure-activity relationship (SAR) around the binding modes of representative compound 2 in complex with hMAO B and hAChE enzymes, revealing inhibitor/protein key contacts and the likely molecular rationale for enzyme selectivity. Compound 2 was also demonstrated to be a strong inhibitor of Aβ42 aggregation, with potency comparable to quercetin. Accordingly, atomic force microscopy (AFM) revealed that the most promising naphthoquinones 2 and 5 and anthraquinones 11 and 12 were able to impair Aβ42 fibrillation, deconstructing the morphologies of its fibrillar aggregates. Moreover, the same compounds exerted a moderate neuroprotective effect against Aβ42 toxicity in primary cultures of cerebellar granule cells. Therefore, our findings demonstrate that these molecules may represent valuable chemotypes toward the development of promising candidates for AD therapy.
N-skatyltryptamines-dual 5-ht6r/d2r ligands with antipsychotic and procognitive potential
Bojarski, Andrzej J.,Bugno, Ryszard,Cie?lik, Paulina,Duszyńska, Beata,Handzlik, Jadwiga,Hogendorf, Adam S.,Hogendorf, Agata,Kaczorowska, Katarzyna,Kurczab, Rafa?,Latacz, Gniewomir,Lenda, Tomasz,Sata?a, Grzegorz,Staroń, Jakub,Szewczyk, Bernadeta
, (2021/08/17)
A series of N-skatyltryptamines was synthesized and their affinities for serotonin and dopamine receptors were determined. Compounds exhibited activity toward 5-HT1A, 5-HT2A, 5-HT6, and D2 receptors. Substitution patterns resulting in affinity/activity switches were identified and studied using homology modeling. Chosen hits were screened to determine their metabolism, permeability, hepatotoxicity, and CYP inhibition. Several D2 receptor antagonists with additional 5-HT6R antagonist and agonist properties were identified. The former combination resembled known antipsychotic agents, while the latter was particularly interesting due to the fact that it has not been studied before. Selective 5-HT6R antagonists have been shown previously to produce procognitive and promnesic effects in several rodent models. Administration of 5-HT6R agonists was more ambiguous-in naive animals, it did not alter memory or produce slight amnesic effects, while in rodent models of memory impairment, they ameliorated the condition just like antagonists. Using the identified hit compounds 15 and 18, we tried to sort out the difference between ligands exhibiting the D2R antagonist function combined with 5-HT6R agonism, and mixed D2/5-HT6R antagonists in murine models of psychosis.
61-54-1 Process route
-
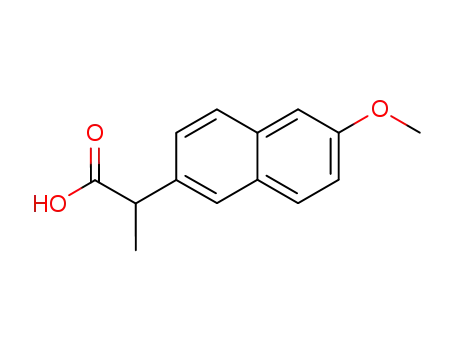
-
23981-80-8
naproxen

-
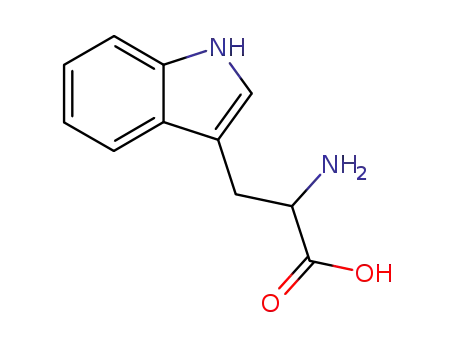
-
54-12-6,27732-43-0,80206-30-0,27813-82-7
Trp

-
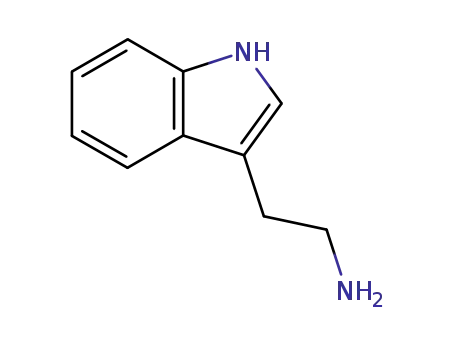
-
61-54-1
tryptamine

-
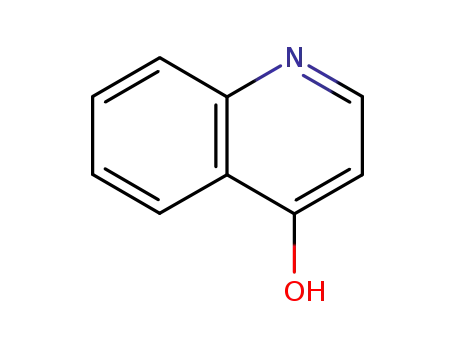
-
611-36-9
quinolin-4-ol
| Conditions | Yield |
|---|---|
|
With
Saccharomyces cerevisiae wild type strain V328;
for 0.333333h;
UV-irradiation;
|
-
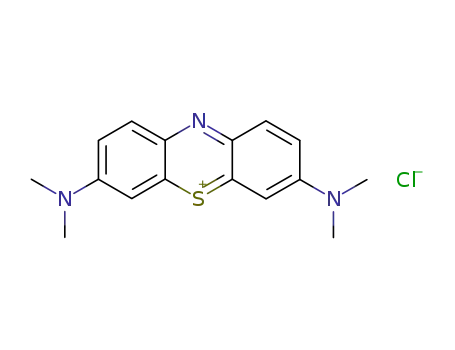
-
152071-32-4,105504-42-5,121067-62-7,12262-49-6,167498-52-4,6476-03-5,97130-83-1
methylene blue

-

-
54-12-6,27732-43-0,80206-30-0,27813-82-7
Trp

-

-
61-54-1
tryptamine

-

-
611-36-9
quinolin-4-ol

-
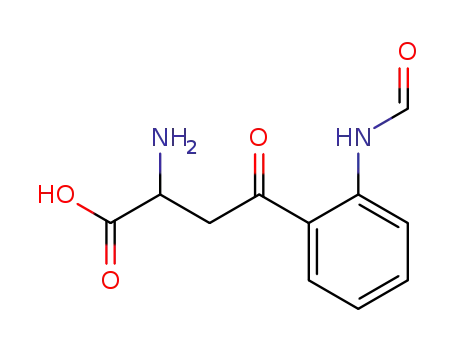
-
1022-31-7,3978-11-8
2-amino-4-(2-formamidophenyl)-4-oxo-butanoic acid

-
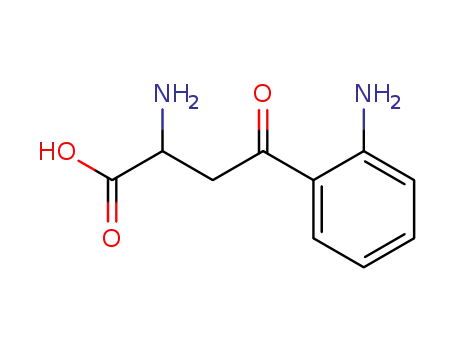
-
343-65-7
Kynurenine
| Conditions | Yield |
|---|---|
|
With
Saccharomyces cerevisiae wild type strain V328;
for 0.333333h;
UV-irradiation;
|
61-54-1 Upstream products
-
771-51-7
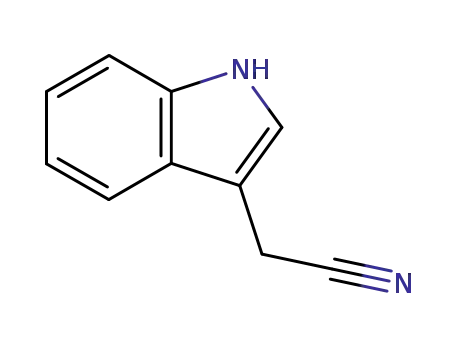
indole-3-acetonitrile
-
3389-21-7
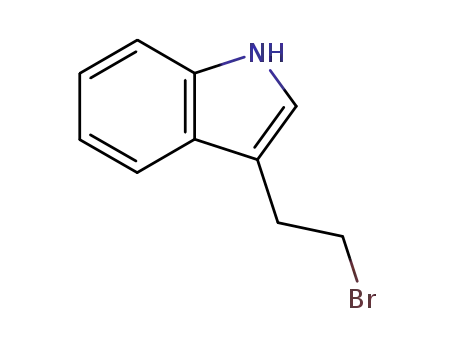
3-(2-bromoethyl)-1H-indole
-
879-37-8
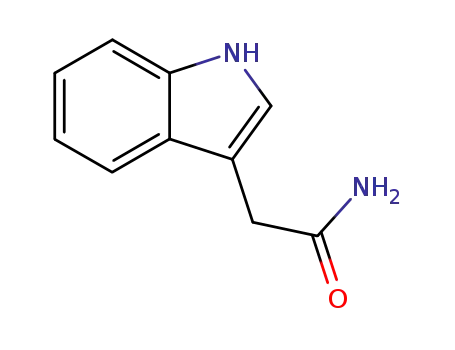
3-indolylacetamide
-
3156-51-2
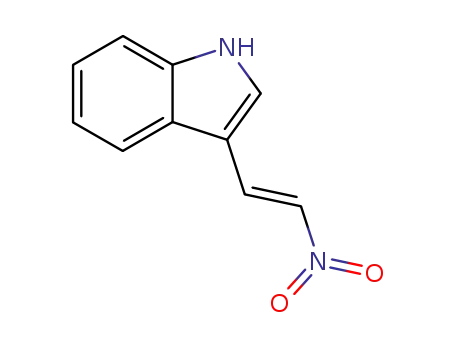
3-[(E)-2-nitroeth-1-enyl]-1H-indole
61-54-1 Downstream products
-
29876-14-0
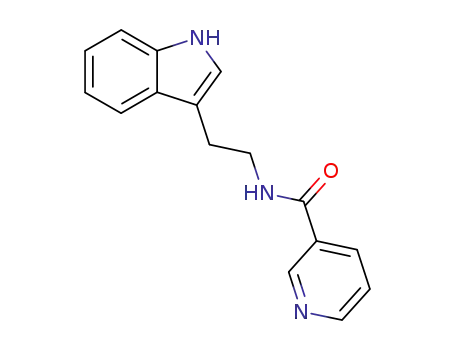
N-[2-(3-indolyl)ethyl]nicotinamide
-
38478-71-6
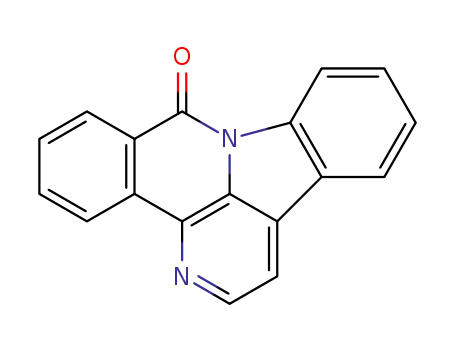
9H-benzo[c]indolo[3,2,1-ij][1,5]naphthyridin-9-one
-
15741-71-6
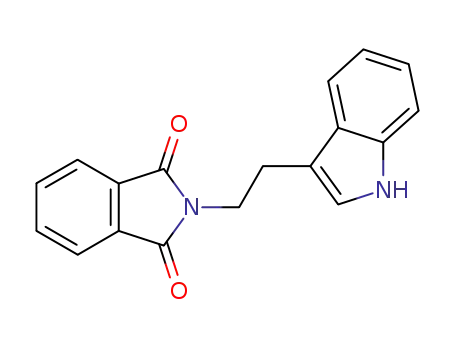
N-phthalimidotryptamine
-
29745-40-2
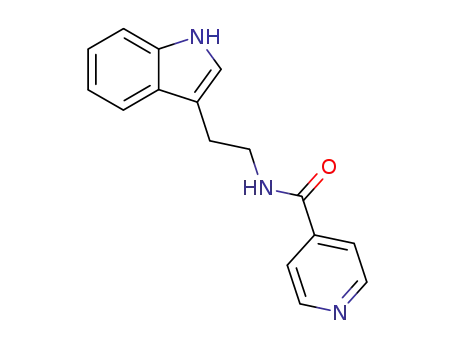
N-(2-(1H-indol-3-yl)ethyl)pyridine-4-carboxamide








