Product Details
81409-90-7 Properties
- Molecular Formula:C26H37N5O2
- Molecular Weight:451.612
- Appearance/Colour:White crystalline solid
- Melting Point:102-104°C
- Refractive Index:1.594
- PKA:13.05±0.46(Predicted)
- PSA:71.68000
- Density:1.156 g/cm3
- LogP:3.52270
81409-90-7 Usage
Description
Cabergoline is a potent, selective, and long-lasting dopamine D2 receptor agonist launched in 1993 in Belgium as a prolactin inhibitor. A single 1 mg dose of cabergoline effectively prevents puerperal lactation for up to 14 days, remarkably superior to other drugs that require a daily regimen. It has a low rate of rebound breast activity and good tolerability. Cabergoline is also in clinical trials for Parkinson's disease, breast cancer, and hyperprolactinaemia.
Chemical Properties
White Crystalline Solid
Originator
Kabi Pharmacia (Sweden)
Uses
A dopamine D2-receptor agonist.
Uses
receptor stimulant
Uses
Cabergoline is an ergot derivative and a dopamine D2-receptor agonist (1,2,3). It inhibits the secretion of prolactin and growth hormone.
Definition
ChEBI: An N-acylurea that is (8R)-ergoline-8-carboxamide in which the hydrogen attached to the piperidine nitrogen (position 6) is substituted by an allyl group and the hydrogens attached to the carboxamide nitrogen are substit ted by a 3-(dimethylamino)propyl group and an N-ethylcarbamoyl group. A dopamine D2 receptor agonist, cabergoline is used in the management of Parkinson's disease and of disorders associated with hyperprolactinaemia.
Manufacturing Process
A mixture of 6-(2-propenyl)-8β-carboxy-ergoline and N-(3- dimethylaminopropyl)-N-ethyl carbodiimide in tetrahydrofuran were refluxed, with stirring and under nitrogen, for 24 h. The resultant solution was evaporated in vacuo to dryness and the residue taken up with chloroform and 5% sodium hydroxide solution. The organic phase was separated, dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was chromatographed on silica (eluant chloroform with 1% methanol) to give the title compound N-(3-(dimethylamino)propyl)-N-((ethylamino)carbonyl)-8β- carboxamide-6-(2-propenyl)ergoline.
Brand name
Dostinex (Pharmacia & Upjohn).
Therapeutic Function
Prolactin inhibitor
General Description
Cabergoline, (6aR,9R,10aR)-7-allyl-N-(3-(dimethylamino)propyl)-N-(ethylcarbamoyl)-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide (Dostinex), is a white powder soluble inalcohol, chloroform, and N,N-dimethylformamide; slightlysoluble in acidic solutions and in n-hexane; and insoluble inwater. Following oral administration, peak plasma concentrationsare reached within 2 to 3 hours. Cabergoline ismoderately bound to plasma proteins in a concentrationindependentmanner. The absolute bioavailability of cabergolineis unknown. Cabergoline is extensively metabolizedby the liver, predominantly via hydrolysis of the acylureabond of the urea moiety. CYP450 metabolism appears to beminimal. The major metabolites identified thus far do not contribute to the therapeutic effect of cabergoline. Lessthan 4% is excreted unchanged in the urine. Fecal excretionrepresents the main route of cabergoline elimination. Thereare no reports of interactions of cabergoline with other antiparkinsonianagents. Clarithromycin may elevate theplasma concentration of cabergoline by the inhibition ofboth CYP3A4 and P-glycoprotein.Cabergoline is a potentD2 receptor agonist and is indicated for the treatment ofhyperprolactinemic disorders, either idiopathic or causedby pituitary adenomas.
Biological Activity
Selective D 2 -like dopamine receptor agonist (K i values are 0.7, 1.5, 9.0 and 165 nM for D 2 , D 3 , D 4 and D 5 receptors respectively) that also displays high affinity for several serotonin receptor subtypes (K i = 1.2-20.0 nM for 5-HT 1A , 5-HT 1D , 5-HT 2A and 5-HT 2B ). Inhibits secretion of prolactin and growth hormone and reverses levodopa-induced dyskinesias in Parkinsonian monkeys.
Biochem/physiol Actions
Cabergoline, a lysergic acid amide derivative, is a potent dopamine D2 receptor agonist. It also acts on dopamine receptors in lactophilic hypothalamus cells to suppress prolactin production in the pituitary gland. It has been used for monotherapy of Parkinson′s disease in the early phase; combination therapy, together with levodopa and a decarboxylase inhibitor such as carbidopa, in progressive-phase Parkinson′s disease and adjunctive therapy of prolactin-producing pituitary gland tumors (microprolactinomas).
Clinical Use
Endocrine disorders Adjunct to levodopa (with a decarboxylase inhibitor) in Parkinson’s disease Inhibition / suppression of lactation
Veterinary Drugs and Treatments
For dogs, cabergoline may be useful for inducing estrus, treatment of primary or secondary anestrus, pseudopregnancy, and pregnancy termination in the second half of pregnancy. Cabergoline may be useful in treating some cases of pituitary-dependent hyperadrenocorticism (Cushing’s). In cats, cabergoline, with or without a prostaglandin, may be useful for pregnancy termination, particularly earlier in pregnancy. Preliminary work has been done in psittacines (primarily Cockatiels) for adjunctive treatment of reproductive-related disorders, particularly persistent egg laying. In humans, cabergoline is indicated for the treatment of disorders associated with hyperprolactenemia or the treatment of Parkinson’s disease.
in vitro
receptor binding studies have demonstrated that cabergoline has high in vitro selectivity and affinity for the subtype d2 of the dopamine receptor. in rat anterior pituitary cells, the concentration of cabergoline required to inhibit prl secretory activity by 50% were 0.1 nmol/l [1].
in vivo
in various animal models, cabergoline markedly reduced plasma prl levels in vivo after single or multiple doses, and the prl-lowering effects appeared 2 - 8 h after administration lasting for 72 h or longer. in addition, a single dose of cabergoline 0.6 mg/kg to rats, inhibited the serum levels of prl for 6 days significantly [1].
Drug interactions
Potentially hazardous interactions with other drugs None known
IC 50
0.1 nm
Metabolism
Cabergoline is subject to first-pass metabolism and is extensively metabolised to several metabolites that do not appear to contribute to its pharmacological activityCabergoline is mainly eliminated via the faeces (72%); a small proportion is excreted in the urine (18%).
references
[1] annamaria colao, gaetano lombardi & lucio annunziato. cabergoline. exp. opin. pharmacother. (2000) 1(3):555-574
InChI:InChI=1/C26H37N5O2/c1-5-11-30-17-19(25(32)31(26(33)27-6-2)13-8-12-29(3)4)14-21-20-9-7-10-22-24(20)18(16-28-22)15-23(21)30/h5,7,9-10,16,19,21,23,28H,1,6,8,11-15,17H2,2-4H3,(H,27,33)/t19-,21?,23-/m1/s1
81409-90-7 Relevant articles
New and efficient process for the preperation of cabergoline and its intermediates
-
Page/Page column 12, (2008/12/08)
This invention relates to a new and efficient process for the production of dopamine agonists such as Cabergoline and the intermediates thereof.
New crystal form of cabergoline
-
Page/Page column 7, (2008/12/07)
The present invention relates to a new needle-form crystalline cabergoline form L, its preparation from halogenated aromatic solvents and aliphatic hydrocarbons and its use in pharmaceutical composition.
PROCESS FOR THE PREPARATION OF CRYSTAL FORMS OF CABERGOLINE VIA NOVEL STABLE SOLVATES OF CABERGOLINE
-
Page/Page column 10, (2008/12/05)
The present invention relates to a process for producing cabergoline crystal forms. The process comprises preparation of the desired solvate of cabergoline from a solution of cabergoline in chloroaromatic solvents. Afterwards cabergoline crystal form is prepared by recovery from a solvate of cabergoline by drying or with desolvating in a solvent media.
PROCESS FOR THE PREPARATION OF AMORPHOUS CABERGOLINE
-
Page/Page column 4,5, (2008/12/07)
The present invention relates to processes for the preparation of amorphous cabergoline by agitated thin film drying or spray drying.
81409-90-7 Process route
-

-
153415-36-2
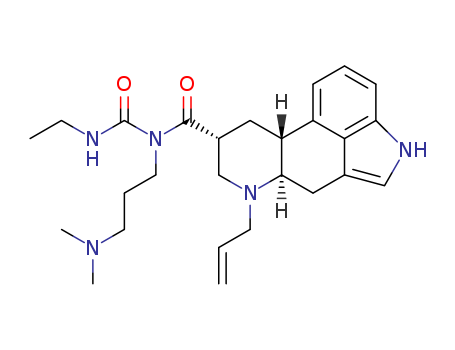
6-Norcabergoline

-
-
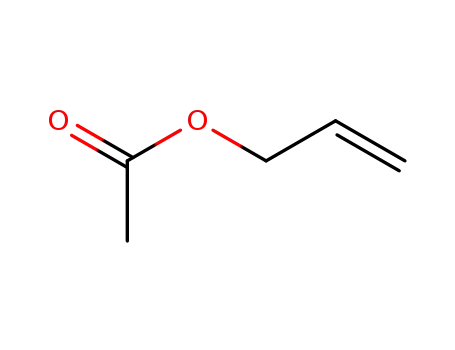
591-87-7
Allyl acetate

-
-
81409-90-7,856676-33-0
cabergoline
| Conditions | Yield |
|---|---|
|
tetrakis(triphenylphosphine) palladium(0);
In
toluene;
at 20 ℃;
for 2h;
|
92.3% |
-
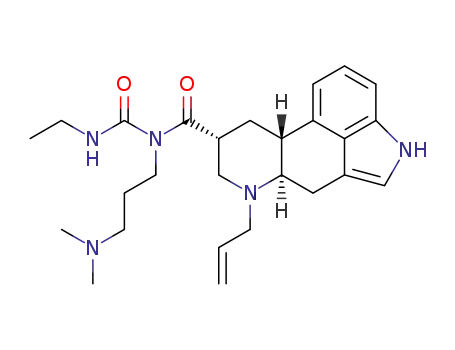
![(6aR,9R,10aR)-7-allyl-N-[3-(dimethylamino)propyl]-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide](/upload/2023/2/f52960f4-dfe8-4f52-8180-859522405699.png)
-
85329-86-8
(6aR,9R,10aR)-7-allyl-N-[3-(dimethylamino)propyl]-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide

-
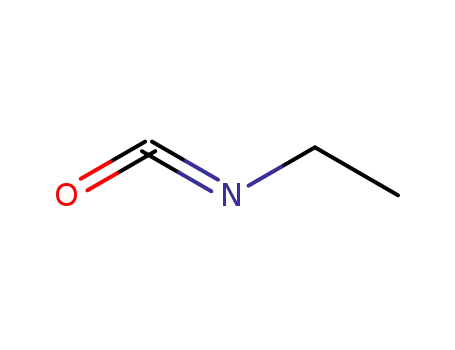
-
109-90-0
ethyl isocyanate

-

-
81409-90-7,856676-33-0
cabergoline
| Conditions | Yield |
|---|---|
|
(6aR,9R,10aR)-7-allyl-N-[3-(dimethylamino)propyl]-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide;
With
trimethylsilyl trifluoromethanesulfonate; triethylamine;
In
dichloromethane;
at -2 - 18 ℃;
for 14h;
ethyl isocyanate;
In
dichloromethane;
at 18 ℃;
for 48h;
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran; dichloromethane;
at -2 ℃;
for 4h;
|
68.2% |
81409-90-7 Upstream products
-
25952-53-8
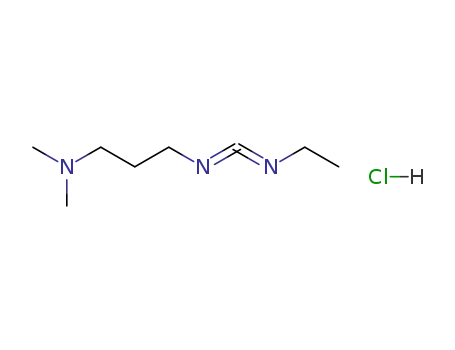
1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride
-
81409-74-7
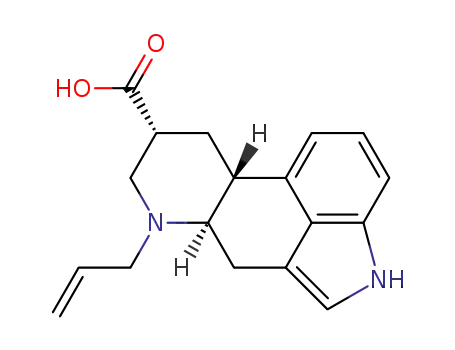
(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylic acid
-
85329-86-8
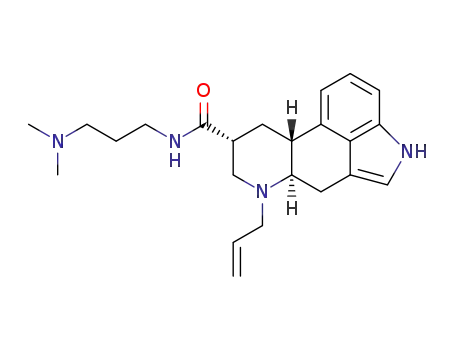
(6aR,9R,10aR)-7-allyl-N-[3-(dimethylamino)propyl]-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide
-
109-90-0

ethyl isocyanate
81409-90-7 Downstream products
-
72821-79-5
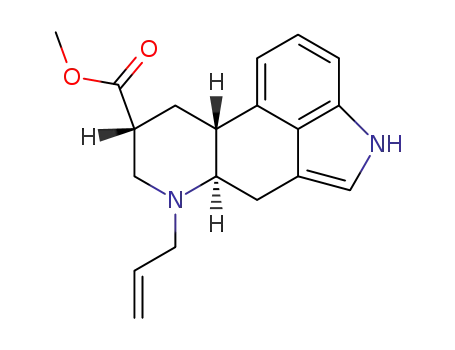
methyl (6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylate
-
81409-74-7

(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylic acid
-
1515864-84-2
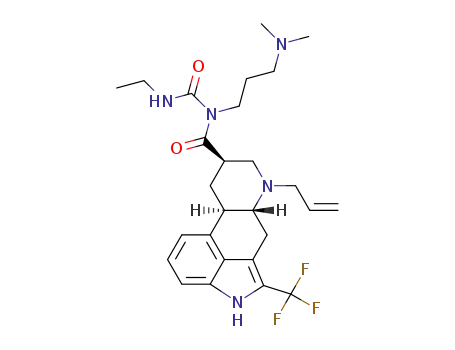
C27H36F3N5O2








