Product Details
1270138-40-3 Properties
- Molecular Formula:C22H30N4O
- Molecular Weight:366.4998
- Boiling Point:534.7±50.0 °C(Predicted)
- PKA:6.18±0.10(Predicted)
- PSA:48.47000
- Density:1.134±0.06 g/cm3(Predicted)
- LogP:3.44640
1270138-40-3 Usage
Description
NSI-189 is an investigational drug being developed by Neuralstem Inc., a biopharmaceutical company. NSI-189 is a small molecule in clinical development for major depressive disorder (MDD) and in preclinical development for Angelman syndrome, irradiation-induced cognitive impairment, Type 1 and Type 2 diabetes, and stroke. NSI-189 was studied in phase I and II clinical trials for the treatment of major depressive disorder. As of July 2017, NSI-189 has failed to meet effectiveness outcomes for depression in phase II trials.
Uses
NSI-189 is an experimental, potential antidepressant, for the treatment for major depressive disorder (MDD), as well as for cognitive impairment and neurodegeneration.
Side effects
NSI 189 is not approved by the FDA, and there is not enough scientific information available to verify its safety. To lessen any potential side effects, it is advisable to only take the lowest possible dose of the compound at first in order to judge how it will affect the body. The possible side effects of this compound include nausea, fatigue, heart palpitations, headache, increased anxiety, visual perception changes, loss of memory, dissociation, decreased appetite, increased heart rate, delirium, and a drop in blood sugar.As there have been no long term studies for this compound, the long term effects and overall safety are unknown. For this reason, it is not suggested to take the compound if an individual is pregnant or breastfeeding. It is also not advisable to take the compound if an individual is taking a prescription medication for the treatment of depression, neuropathy, or if they have low blood sugar.
Mode of action
NSI-189 is a compound that may increase the birth of new neurons (neurogenesis) in the brain area known as the hippocampus. It works by stimulating the growth of new brain cells and nerve cells through a process called neurogenesis. The neurogenesis appears to affect the hippocampus primarily, which is responsible for learning, memory, and emotion. Scientists believe the compound has the potential to treat various neurological and nerve conditions.
Clinical claims and research
NSI-189 completed a phase I clinical trial for MDD in 2011, where it was administered to 41 healthy volunteers.A phase Ib clinical trial for treating MDD in 24 patients started in 2012 and completed in July 2014, with results published in December 2015. In July 2017, it was announced that a phase II clinical trial with 220 patients failed to meet its primary effectiveness endpoint in MDD.Upon the announcement, Neuralstem stock plummeted by 61%.More detailed analysis of the trial results was released in December 2017 and January 2018. The compound has gone through phase I and II clinical trials for its antidepressant properties, but the phase II trial failed to meet effectiveness outcomes. It is currently still being studied as a possible treatment for other conditions.
1270138-40-3 Relevant articles
Synthetic method for antidepressant NSI189 and phosphate thereof
-
Paragraph 0050; 0059; 0060; 0061, (2019/10/01)
The invention discloses a synthetic method for an antidepressant NSI189 and phosphate thereof. The method includes firstly activating 2-chloronicotinic acid A1 adopted as an initial raw material to obtain an acyl chloride compound; then reacting with N-monoprotected substituted piperazine to generate an intermediate A2; after removing the protecting group, reacting the intermediate A2 with a benzyl halide to generate an intermediate A3; reacting benzylamine or a benzylamine derivative A4 having a substitute on the benzene ring with isovaleraldehyde A5 to prepare an intermediate A6; coupling the intermediate A3 and the intermediate A6 to generate an intermediate A7; removing benzyl or derivative of the intermediate A7 under the existence of an inorganic acid or an oxidation agent to generate a compound A8; salifying the compound A8 with concentrated phosphoric acid to form a compound A9. In the method, all steps are easy to operate, reaction reagents are cheap and easily available, theproduct yield is high, the production cost is low, and no flammable combustible hazardous reagent or production operation is adopted, so that the method is suitable for large-scale industrial production and the method has a wide production application prospect.
Synthesis of a neurostimulative piperazine
-
Page/Page column 13, (2016/03/26)
The invention describes an improved synthesis for piperazine derivatized with nicotinic acid and a benzyl moiety. The product compounds are useful for treatment of neurological conditions.
1270138-40-3 Process route
-
-
C30H38N4O2

-
![(4-benzylpiperazin-1-yl)-[2-(3-methyl-butylamino)pyridin-3-yl] methanone](/upload/2023/2/a96d979f-c3cd-4c10-8a66-5322b469682f.png)
-
1270138-40-3
(4-benzylpiperazin-1-yl)-[2-(3-methyl-butylamino)pyridin-3-yl] methanone
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 2h;
Reflux;
|
75% |
-
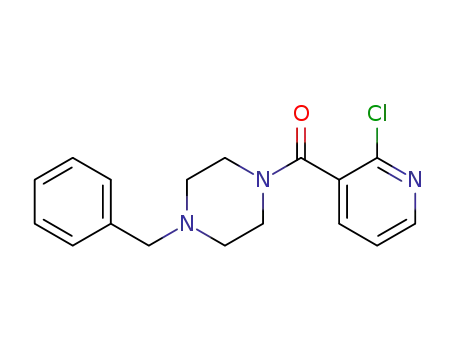
-
544428-67-3
C17H18ClN3O

-
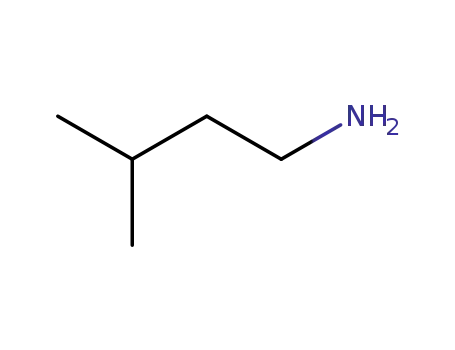
-
107-85-7
1-amino-3-methylbutane

-
![(4-benzylpiperazin-1-yl)-[2-(3-methyl-butylamino)pyridin-3-yl] methanone](/upload/2023/2/a96d979f-c3cd-4c10-8a66-5322b469682f.png)
-
1270138-40-3
(4-benzylpiperazin-1-yl)-[2-(3-methyl-butylamino)pyridin-3-yl] methanone
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
at 85 ℃;
for 19h;
Temperature;
|
7.78 g |
1270138-40-3 Upstream products
-
49609-84-9
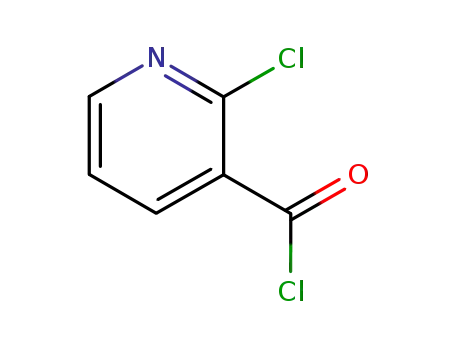
2-Chloronicotinoyl chloride
-
551921-02-9
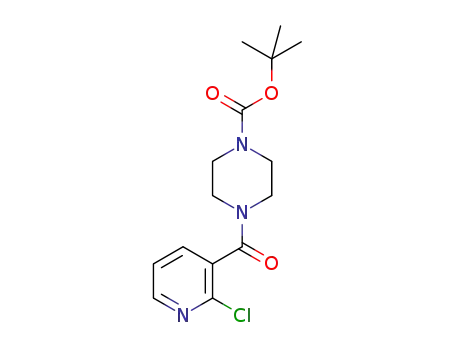
1-(2-chloronicotinoyl)-4-tert-butoxycarbonylpiperazine
-
544428-67-3

C17H18ClN3O
-
107-85-7
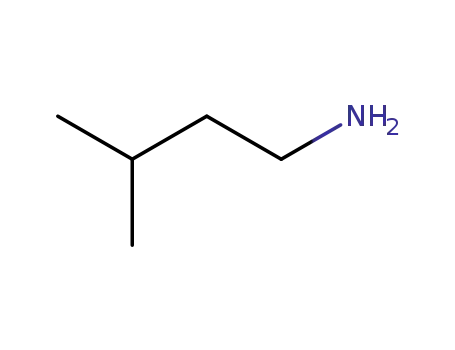
1-amino-3-methylbutane








