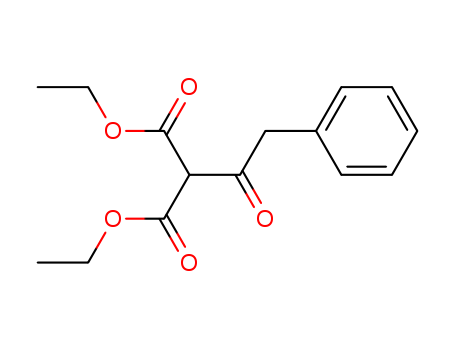
Diethyl (phenylacetyl) Malonate 20320-59-6
- CasNo:20320-59-6
- Molecular Formula:
- Purity:
- Molecular Weight:
Product Details
20320-59-6 Properties
- Molecular Formula:C15H18O5
- Molecular Weight:278.305
- Boiling Point:120 °C(Press: 0.01 Torr)
- PKA:8.76±0.59(Predicted)
- PSA:69.67000
- Density:1.148±0.06 g/cm3(Predicted)
- LogP:1.54060
20320-59-6 Usage
Physical properties
Diethyl(phenylacetyl)malonate has a boiling point of 120 °C. Its density is predicted to be 1.148±0.06 g/cm3.
Uses
Organic synthesis intermediates.
Preparation
The anhydrous ethanol co-vaporized with diethyl phthalate and sodium metal is added dropwise to the mixture of diethyl malonate, carbon tetrachloride and magnesium, heated to start the reaction, and the temperature is controlled to smooth the reaction. Then add anhydrous diethyl ether, heat for 1h, add the diethyl ether solution of phenylacetyl chloride (add slowly, not to make the reaction too intense). After the reaction is completed, cool and add water, separate the oil layer, and evaporate the diethyl ether under reduced pressure to obtain diethyl(phenylacetyl)malonate.
20320-59-6 Relevant articles
Comprehensive study on excited state intramolecular proton transfer in 2-(benzo[d]thiazol-2-yl)-3-methoxynaphthalen-1-ol and 2-(benzo[d]thiazol-2-yl)naphthalene-1,3-diol: Effect of solvent, aggregation, viscosity and TDDFT study
Warde, Umesh,Nagaiyan, Sekar
, p. 33 - 43 (2017)
Three compounds DMT, MMT and DHT having dimehtoxy, mono-hydroxy and di-hydroxy groups respectively were prepared. Environmental interactions dependent photophysical properties especially excited state intramolecular proton transfer (ESIPT), solvatochromism, aggregation induced emission enhancement (AIEE) and viscosity dependent emission characteristics were studied. The effect of solvent polarity on ESIPT dynamics were studied using UV–vis and emission spectroscopy along with aggregation induced enhanced emission and viscosity effect. MMT and DHT showed unexpected and contrasting behavior in the solvents which are in good agreement with the density functional theory and time dependent density functional theory findings. Aggregation and viscosity study showed that cis-keto emission increased drastically in the aggregate state and highly viscous state due to restricted intramolecular rotation increasing the population of required cis enol rotamer (E). The study directs the potential applications of such molecules for advanced optoelectronics and viscosity based investigations.
The Interrupted Pummerer Reaction in a Sulfoxide-Catalyzed Oxidative Coupling of 2-Naphthols
He, Zhen,Pulis, Alexander P.,Procter, David J.
supporting information, p. 7813 - 7817 (2019/05/15)
A benzothiophene S-oxide catalyst, generated in situ by sulfur oxidation with H2O2, mediates the oxidative coupling of 2-naphthols. Key to the catalytic process is the capture and inversion of reactivity of a 2-naphthol partner, using an interrupted Pummerer reaction of an unusual benzothiophene S-oxide, followed by subsequent coupling with a second partner. The new catalytic manifold has been showcased in the synthesis of the bioactive natural products, (±)-nigerone and (±)-isonigerone. Although Pummerer reactions are used widely, their application in catalysis is rare, and our approach represents a new catalytic manifold for metal-free C?C bond formation.
INDOLIN-2-ONE DERIVATIVES AS PROTEIN KINASE INHIBITORS
-
Paragraph 0283, (2013/11/05)
A novel class of indoline-2-one derivatives are disclosed. These compounds are protein kinase inhibitors which are useful for treating hyperproliferative diseases such as cancer.
Novel Inhibitors of Hepatitis C Virus Replication
-
Page/Page column 52-53, (2009/10/21)
The embodiments provide compounds of the general Formula I, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating a hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
20320-59-6 Process route
-
-
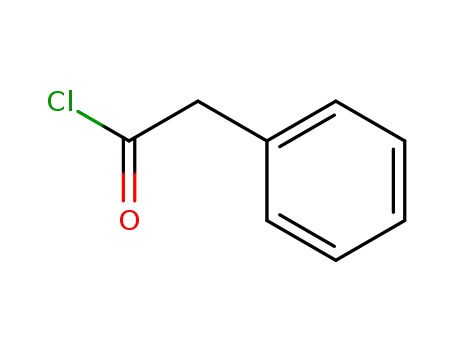
103-80-0
phenylacetyl chloride

-
-
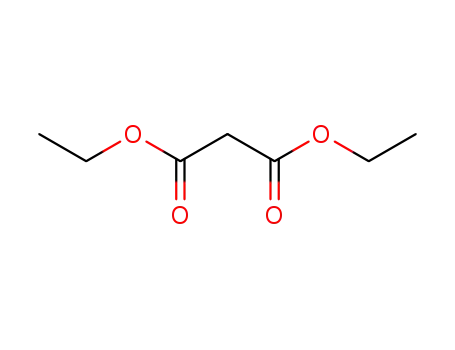
105-53-3
diethyl malonate

-
-
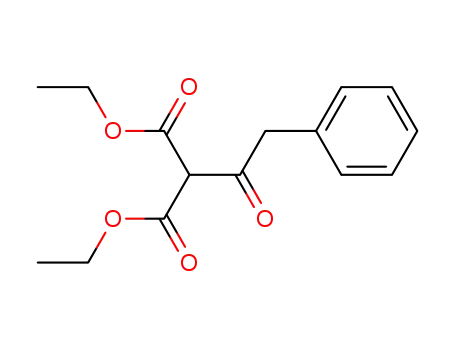
20320-59-6
diethyl 2-(2-phenylacetyl)malonate
| Conditions | Yield |
|---|---|
|
diethyl malonate;
With
ethanol; magnesium;
In
tetrachloromethane; toluene;
for 1h;
Heating;
phenylacetyl chloride;
In
tetrachloromethane; toluene;
at 20 ℃;
for 1h;
|
90% |
|
With
chloroform; magnesium;
|
|
|
With
magnesium;
|
|
|
With
sodium;
|
|
|
Multistep reaction;
(i) Mg, EtOH, (ii) /BRN= 742254/;
|
|
|
diethyl malonate;
With
triethylamine; magnesium chloride;
In
acetonitrile;
at 0 ℃;
for 0.5h;
Inert atmosphere;
phenylacetyl chloride;
In
acetonitrile;
at 20 ℃;
|
|
|
With
magnesium;
In
ethanol; toluene;
at 80 ℃;
|
|
|
diethyl malonate;
With
sodium hydride;
In
tetrahydrofuran;
for 1h;
phenylacetyl chloride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1h;
|
489 mg |
-
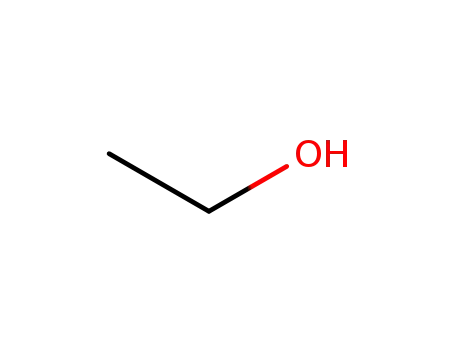
-
64-17-5
ethanol

-
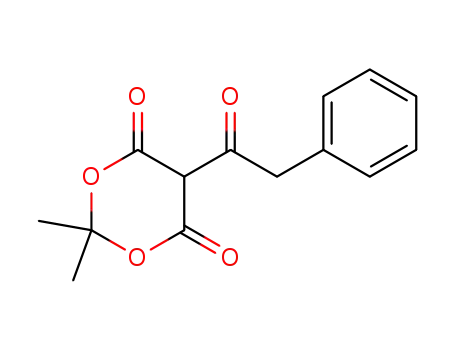
-
74965-87-0
2,2-dimethyl-5-(2-phenylacetyl)-1,3-dioxane-4,6-dione

-

-
20320-59-6
diethyl 2-(2-phenylacetyl)malonate
| Conditions | Yield |
|---|---|
|
for 3h;
Reflux;
|
20320-59-6 Upstream products
-
996-82-7
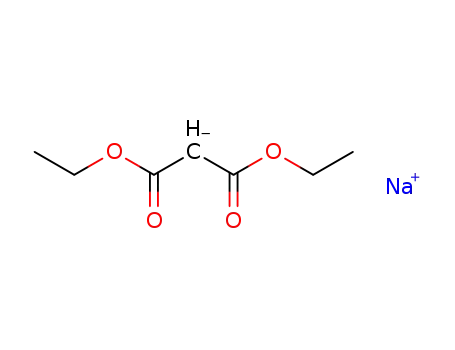
sodium diethylmalonate
-
103-80-0

phenylacetyl chloride
-
105-53-3

diethyl malonate
-
570-08-1
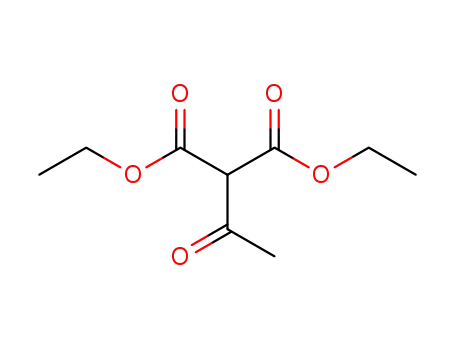
diethyl acetylmalonate
20320-59-6 Downstream products
-
621-06-7
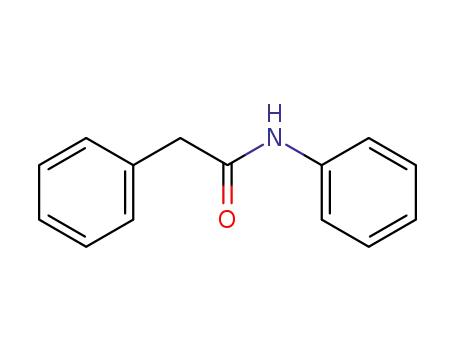
2,N-diphenylacetamide
-
20280-94-8
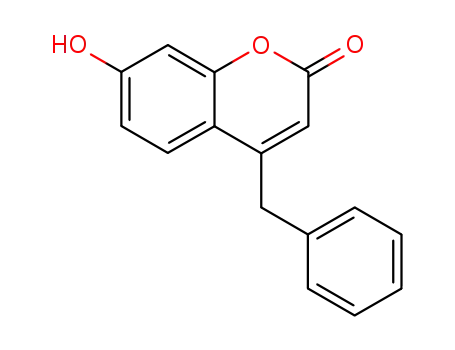
4-benzyl-7-hydroxycoumarin
-
20597-30-2
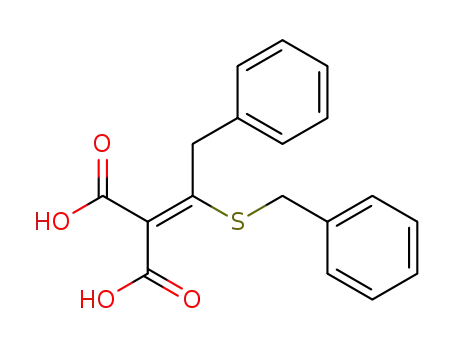
2-Benzylmercapto-3-phenyl-1-propen-1,1-dicarbonsaeure
-
101981-93-5
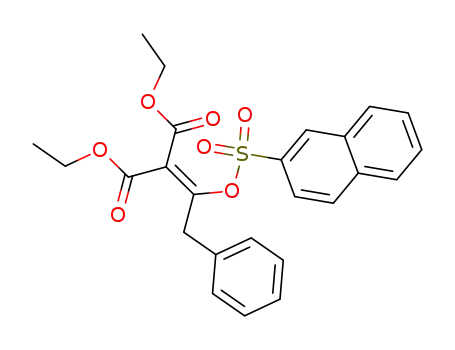
Naphthalin-2-sulfonsaeure-<1-benzyl-2,2-diaethoxycarbonyl-vinyl-ester>








