Teriparatide Acetate 52232-67-4
- CasNo:52232-67-4
- Molecular Formula:C181H291N55O51S2
- Purity:
- Molecular Weight:
Product Details
|
52232-67-4 Name |
|
|
Name |
Teriparatide Acetate |
|
Synonym |
PARATHYROID HORMONE HUMAN: FRAGMENT 1-34;PARATHYROID HORMONE (HUMAN, 1-34);PARATHYROID HORMONE (1-34), HUMAN;PTH (1-34) (HUMAN);PTH (HUMAN, 1-34);TERIPARATIDE;Teriparatide acetate;SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF |
|
52232-67-4 Biological Activity |
|
|
Description |
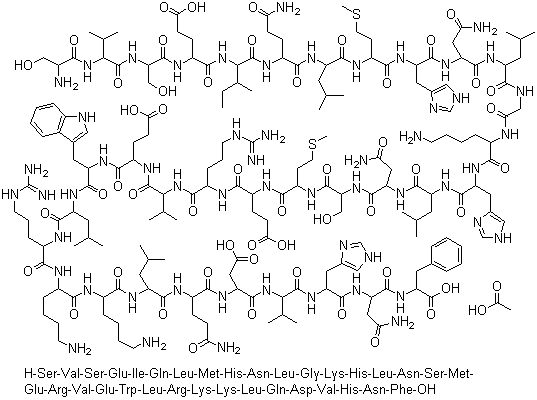
Teriparatide is a PHT agonist, with an IC50 of 2 nM in HEK293 cells. Sequence: Ser-Val-Ser-Glu-Ile-Gln-Leu-Met-His-Asn-Leu-Gly-Lys-His-Leu-Asn-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg-Lys-Lys-Leu-Gln-Asp-Val-His-Asn-Phe. |
|
Related Catalog |
Signaling Pathways >> Others >> Others Research Areas >> Cancer Peptides |
|
Target |
IC50: 2 nM (PTH)[1]. |
|
In Vivo |
Trabecular bone calcium and dry weight of the distal femur increased significantly in Teriparatide-treated animals. The increase in trabecular calcium compared with vehicle control occurred as early as 1 week after initiation of treatment with a 35% and 45% increase, respectively, for 10 μg/kg and 40 μg/kg Teriparatide. Similar results were observed for trabecular dry weight. After 4 weeks of treatment with 10 mg/kg or 40 mg/kg Teriparatide, trabecular calcium increased significantly by 70% and 123%, respectively, compared with the vehicle and by 73%[1]. The 4-week Teriparatide administration increase the pore ratio, number, and density as well as the cortical area, thickness, and bone mineral content (BMC), without significant influencing the volumetric bone mineral density (BMD). The 4-week Teriparatide administration + 8-week vehicle administration decrease the pore ratio, number, and density as well as the cortical area and thickness, compared with the 4-week Teriparatide administration, but the pore ratio, cortical area, and thickness are still higher compared with the 12-week vehicle administration. The 4-week Teriparatide administration + 8-week higherdose IBN administration increase the cortical area, thickness, BMC, and volumetricBMD and decrease the pore ratio, but not the pore number or density, compared with the 4-week Teriparatide administration + 8-week vehicle administration[2]. |
|
References |
[1]. Frolik CA, et al. Comparison of recombinant human PTH(1-34) (LY333334) with a C-terminally substituted analog of human PTH-related protein(1-34) (RS-66271): In vitro activity and in vivo pharmacological effects in rats. J Bone Miner Res. 1999 Feb;14(2):163-72. [2]. Iwamoto J, et al. Influence of Teriparatide and Ibandronate on Cortical Bone in New Zealand White Rabbits: A HR-QCT Study. Calcif Tissue Int. 2016 Nov;99(5):535-542. |
|
52232-67-4 Chemical & Physical Properties |
|
|
Melting point |
>205°C (dec.) |
|
Molecular Formula |
C181H291N55O51S2 |
|
Molecular Weight |
4177.77000 |
|
Appearance of Characters |
Powder |
|
Storage condition |
-20°C |
|
solubility |
DMSO (Slightly), Water (Slightly) |
|
52232-67-4 Description |
|
Teriparatide is a recombinantform of parathyroid hormone, which is used for the treatmentof osteoporosis in men and postmenopausal women.The N-terminal region possesses 34 amino acids, which areidentical to the biologically active region of the 84-aminoacid sequence of human parathyroid hormone. It has beenshown to act on osteoblasts to stimulate new bone growthand improve bone density. |
|
52232-67-4 Uses |
|
A fragment of human parathyroid hormone (hPTH) peptide sequence containing the 34 N-terminal residues of hPTH. This fragment was also found to be an agonist at PTH1 and PTH2 receptors. |








