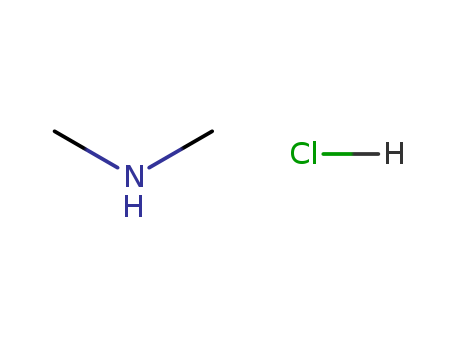
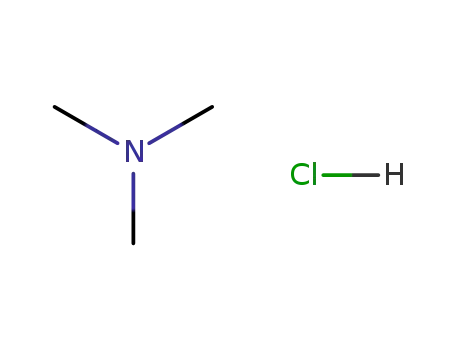
Dimethylamine hydrochloride
- CasNo:506-59-2
- Molecular Formula:C<sub>2</sub>H<sub>7</sub>N<sup>.</sup>HCl
- Purity:
- Molecular Weight:81.5452
Product Details
Chinese Factory Supply Wholesale Dimethylamine hydrochloride 506-59-2 with Cheap Price
- Molecular Formula:C2H7N.HCl
- Molecular Weight:81.5452
- Appearance/Colour:white crystals
- Vapor Pressure:<0.1 hPa (25 °C)
- Melting Point:170-173 ºC (lit.)
- Refractive Index:1.4202 (estimate)
- Boiling Point:6.1 ºC at 760 mmHg
- PSA:12.03000
- Density:0.64 g/cm3
- LogP:1.02850
Dimethylamine hydrochloride(Cas 506-59-2) Usage
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
Crystallise the salt from hot CHCl3 or absolute EtOH. It also recrystallises from MeOH/ether solution. Dry it in a vacuum desiccator over H2SO4, then P2O5. Hygroscopic. [Beilstein 4 IV 132.] |
|
Application |
Dimethylamine hydrochloride is a raw material for organic synthesis. It is also used as a catalyst and magnesium reagent for acetylation analysis.Dimethylamine hydrochloride has been used in the preparation of hexamethylmelamine-methyl-14C. It has also been used to prepare the standard solution of methylamine (MA), dimethylamine (DMA), trimethylamine (TMA), and trimethylamine-N-oxide (TMAO) while determing methylamines and trimethylamine-N-oxide in particulate matter. Dimethylamine hydrochloride is used as an intermediate in the manufacture of pharmaceuticals like ranitidine and metformin, amlodipine. It is used as a precursor of atrazine. It is associated with sodium acetate and used to carry out the Willgerodt- Kindler reaction to prepare amides. Its free base reacts with carbon disulfide to get dimethyldithiocarbamate which is used in rubber vulcanization. It is involved in the synthesis of dimethyl-(1-methyl-pyrrol-2-ylmethyl)-amine by reacting with 1-methyl pyrrole and formaldehyde. |
InChI:InChI=1/C2H7N.ClH/c1-3-2;/h3H,1-2H3;1H
506-59-2 Relevant articles
Dihydrogen bond intermediated alcoholysis of dimethylamine-borane in nonaqueous media
Golub, Igor E.,Gulyaeva, Ekaterina S.,Filippov, Oleg A.,Dyadchenko, Victor P.,Belkova, Natalia V.,Epstein, Lina M.,Arkhipov, Dmitry E.,Shubina, Elena S.
, p. 3853 - 3868 (2015)
Dimethylamine-borane (DMAB) acid/base pr...
Dehydropyrroliumsalze
Gompper, Rudolf,Junius, Martina
, p. 2883 - 2886 (1980)
Reaction of pentachloro-2H-pyrrole with ...
The enthalpies of formation of bis(dimethylamino)cyanophosphine, (dimethylamino)dicyanophosphine, and tricyanophosphine
Al-Maydama, H. M. A.,Finch, Arthur,Gardner, P. J.,Head, A. J.
, p. 575 - 584 (1995)
The standard molar enthalpies of formati...
Studies in Cyclophosphazenes. Part 9. Influence of the Steric Requirements of the Amino-substituents on the Rates of Amination of 2-Amino-2,4,4,6,6-pentachlorocyclotri(λ5-phosphazenes)
Goldschmidt, Jacob M.E.,Licht, Eliahu
, p. 107 - 110 (1981)
Measurements of the rates of amination o...
Copper(II) complexes of tetradentate N2S2 donor sets: Synthesis, crystal structure characterization and reactivity
Sarkar,Patra,Drew,Zangrando,Chattopadhyay
, p. 1 - 6 (2009)
Two mononuclear and one dinuclear copper...
Norbornane-2-spiro-α-cycloalkanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride, norbornane-2-spiro-α-cycloalkanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic acid and ester thereof, method for producing norbornane-2-spiro-α-cycloalkanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride, polyimide obtained by using the same, and method for producing polyimide
-
Page/Page column 55, (2016/10/17)
A norbornane-2-spiro-α-cycloalkanone-α′-...
Stereoselective synthesis of (S)-dapoxetine: A chiral auxiliary mediated approach
Khatik, Gopal L.,Sharma, Ratnesh,Kumar, Varun,Chouhan, Mangilal,Nair, Vipin A.
, p. 5991 - 5993 (2013/10/22)
An imidazolidin-2-one chiral auxiliary m...
Nucleophilic substitution reaction at the nitrogen of arylsulfonamides with phosphide anion
Yoshida, Suguru,Igawa, Kazunobu,Tomooka, Katsuhiko
supporting information, p. 19358 - 19361 (2013/02/22)
A novel nucleophilic substitution reacti...
506-59-2 Process route
-
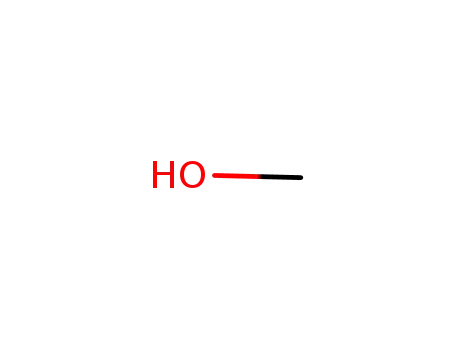
-
67-56-1
methanol

-
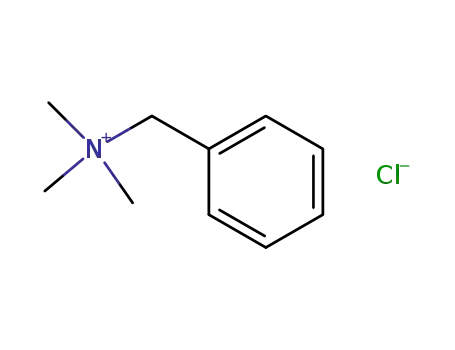
-
56-93-9
benzyltrimethylammonium chloride

-
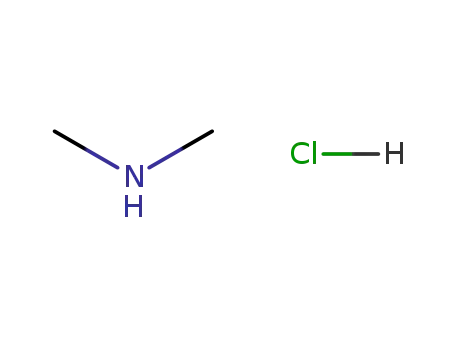
-
506-59-2
N,N-dimethylammonium chloride

-
-
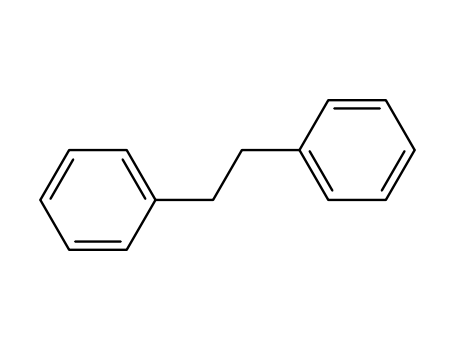
103-29-7
1,1'-(1,2-ethanediyl)bisbenzene

-
-
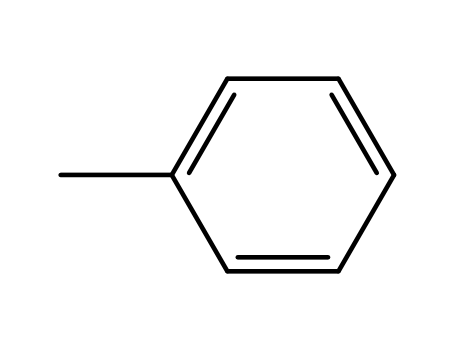
108-88-3,15644-74-3,16713-13-6
toluene

-
-
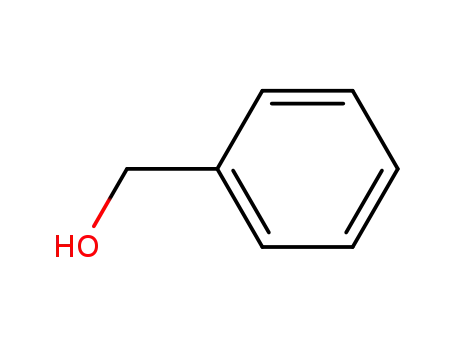
100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With
water;
at 30 ℃;
for 2h;
Further byproducts given;
Irradiation;
|
27 % Chromat. 10 % Chromat. 45 % Chromat. 23 % Chromat. |
-

-
56-93-9
benzyltrimethylammonium chloride

-

-
506-59-2
N,N-dimethylammonium chloride

-
-
593-81-7
trimethylamine hydrochloride

-

-
103-29-7
1,1'-(1,2-ethanediyl)bisbenzene

-

-
108-88-3,15644-74-3,16713-13-6
toluene

-

-
100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With
water;
In
water;
at 30 ℃;
for 2h;
Mechanism;
Irradiation;
253.7 nm;
|
45 % Chromat. 27 % Chromat. 10 % Chromat. 77 % Chromat. 23 % Chromat. |
506-59-2 Upstream products
-
1585-74-6
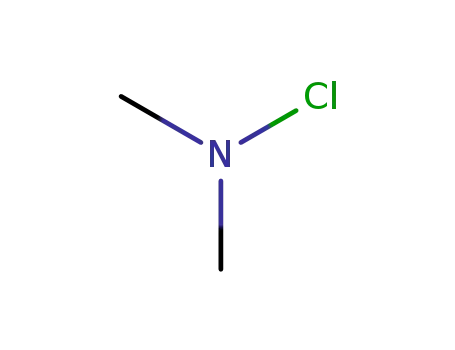
dimethylchloroamine
-
85608-26-0
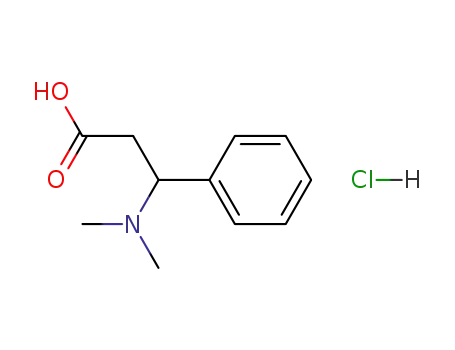
Winterstein acid hydrochloride
-
141-78-6
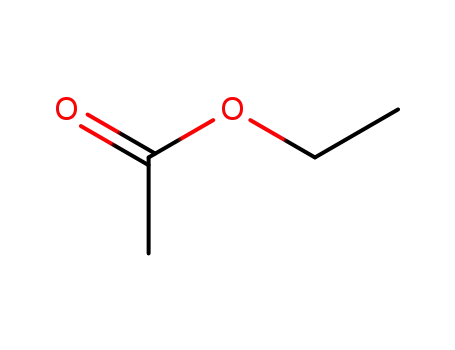
ethyl acetate
-
74-87-3
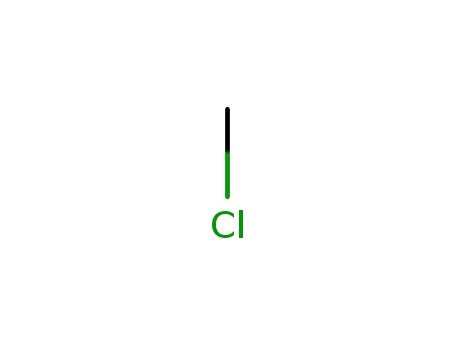
methylene chloride
506-59-2 Downstream products
-
104926-40-1
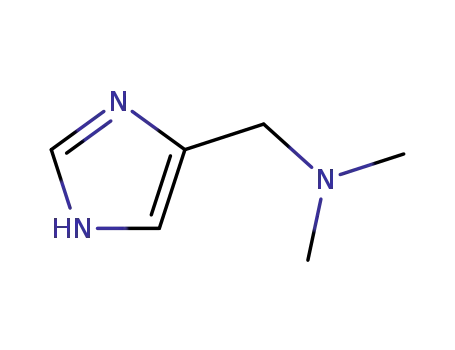
1H-imidazol-4-yl-N,N-dimethylmethanamine
-
56139-76-5
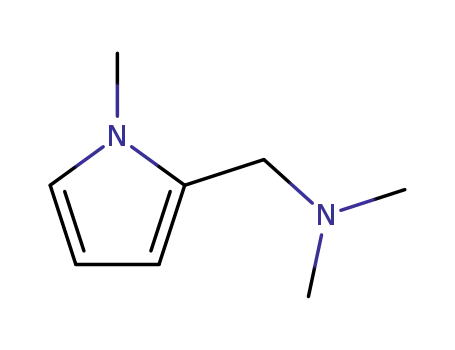
2-(N,N-dimethylaminomethyl)-1-methylpyrrole
-
6304-27-4
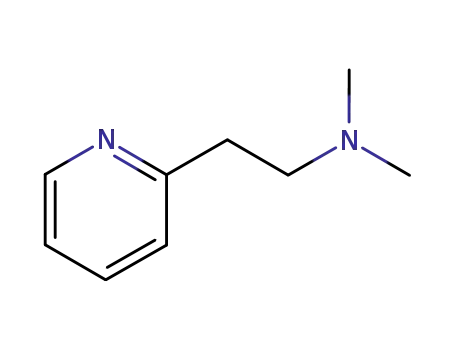
2-<-(dimethylamino)ethyl>pyridine
-
15433-79-1
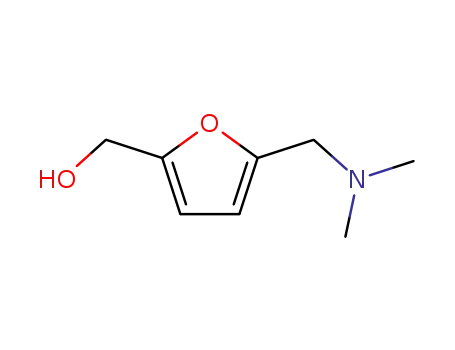
(5-dimethylaminomethyl-furan-2-yl)-methanol








