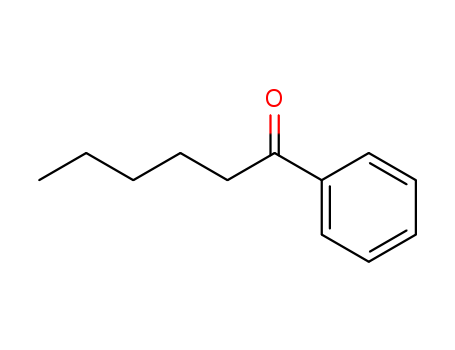
Hexanophenone
- CasNo:942-92-7
- Molecular Formula:C<sub>12</sub>H<sub>16</sub>O
- Purity:
- Molecular Weight:176.258
Product Details
Cosmetics Grade Hexanophenone 942-92-7 For Sale with Good Price
- Molecular Formula:C12H16O
- Molecular Weight:176.258
- Appearance/Colour:clear light yellow liquid after melting
- Vapor Pressure:0.0094mmHg at 25°C
- Melting Point:25-26 °C(lit.)
- Refractive Index:n20/D 1.5105(lit.)
- Boiling Point:265 °C at 760 mmHg
- Flash Point:105.5 °C
- PSA:17.07000
- Density:0.942 g/cm3
- LogP:3.44960
HEXANOPHENONE(Cas 942-92-7) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 44, p. 3585, 1979 DOI: 10.1021/jo01334a033Synthetic Communications, 8, p. 59, 1978 DOI: 10.1080/00397917808062184Tetrahedron Letters, 24, p. 3677, 1983 DOI: 10.1016/S0040-4039(00)88199-X |
InChI:InChI=1/C12H16O/c1-2-3-5-10-12(13)11-8-6-4-7-9-11/h4,6-9H,2-3,5,10H2,1H3
942-92-7 Relevant articles
Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene
Goettmann, Frederic,Fischer, Anna,Antonietti, Markus,Thomas, Arne
, p. 4467 - 4471 (2006)
(Figure Presented) In the footsteps of L...
Rh-Catalyzed Coupling of Aldehydes with Allylboronates Enables Facile Access to Ketones
Zhang, Kezhuo,Huang, Jiaxin,Zhao, Wanxiang
supporting information, (2022/02/21)
We present herein a novel strategy for t...
Nickel-Mediated Photoreductive Cross Coupling of Carboxylic Acid Derivatives for Ketone Synthesis**
Brauer, Jan,Quraishi, Elisabeth,Kammer, Lisa Marie,Opatz, Till
supporting information, p. 18168 - 18174 (2021/11/30)
A simple visible light photochemical, ni...
Metal- And additive-free C-H oxygenation of alkylarenes by visible-light photoredox catalysis
García Manche?o, Olga,Kuhlmann, Jan H.,Pérez-Aguilar, María Carmen,Piekarski, Dariusz G.,Uygur, Mustafa
supporting information, p. 3392 - 3399 (2021/05/21)
A metal- and additive-free methodology f...
Selective catalytic synthesis of α-alkylated ketones and β-disubstituted ketones via acceptorless dehydrogenative cross-coupling of alcohols
Bhattacharyya, Dipanjan,Sarmah, Bikash Kumar,Nandi, Sekhar,Srivastava, Hemant Kumar,Das, Animesh
supporting information, p. 869 - 875 (2021/02/06)
Herein, a phosphine-free pincer rutheniu...
942-92-7 Process route
-
-
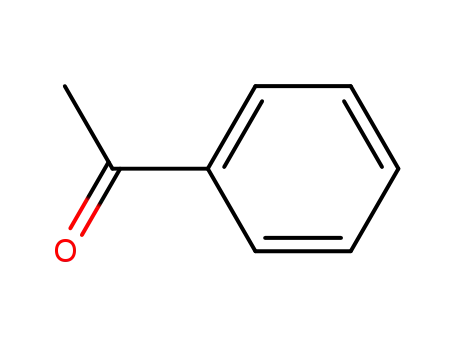
98-86-2
acetophenone

-
-
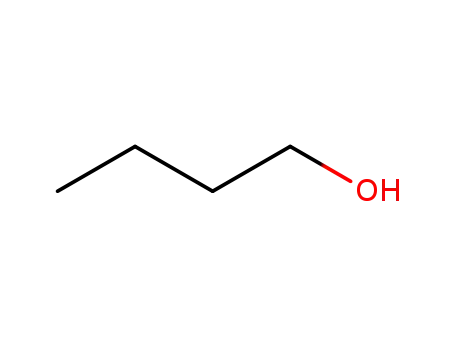
71-36-3
butan-1-ol

-
-
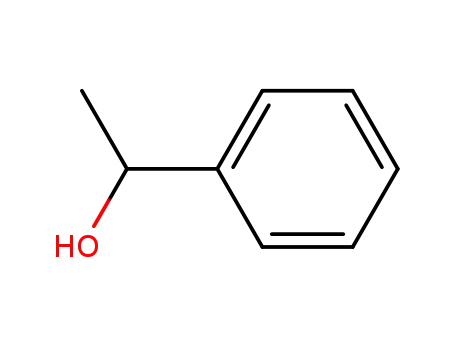
98-85-1,13323-81-4
1-Phenylethanol

-
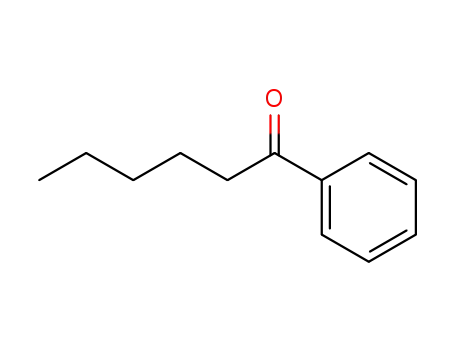
-
942-92-7
caprophenone
| Conditions | Yield |
|---|---|
|
With
tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; caesium carbonate; triphenylphosphine;
In
toluene;
at 140 ℃;
for 48h;
Inert atmosphere;
Schlenk technique;
Sealed tube;
Green chemistry;
|
55% 30 %Spectr. |
|
With
[2,2]bipyridinyl; rhodium(III) chloride; sodium hydroxide;
In
water;
at 110 ℃;
Reagent/catalyst;
Temperature;
Inert atmosphere;
Microwave irradiation;
|
38 %Chromat. 14 %Chromat. |
-

-
98-86-2
acetophenone

-

-
71-36-3
butan-1-ol

-

-
98-85-1,13323-81-4
1-Phenylethanol

-

-
942-92-7
caprophenone

-
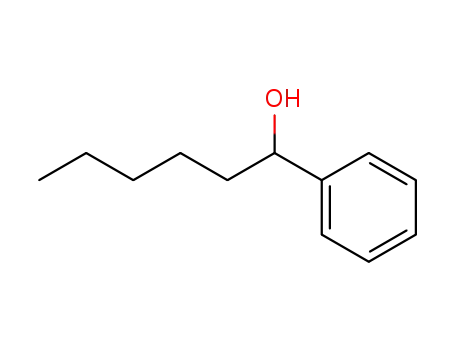
-
4471-05-0
1-phenylhexan-1-ol
| Conditions | Yield |
|---|---|
|
With
cis,cis,trans-[RuCl2{κ2-(P,N)-2-Ph2PC6H4CH=NOH}2]; potassium hydroxide;
In
toluene;
at 120 ℃;
for 3h;
Sealed tube;
Inert atmosphere;
|
60% 20 %Chromat. 6 %Chromat. |
|
With
ruthenium-carbon composite; sodium dodecyl-sulfate; lithium hydroxide;
In
water;
at 140 ℃;
Inert atmosphere;
Microwave irradiation;
|
20 %Chromat. 19 %Chromat. 8 %Chromat. |
942-92-7 Upstream products
-
110-86-1
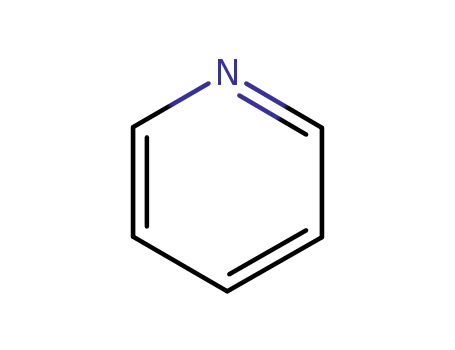
pyridine
-
21726-36-3
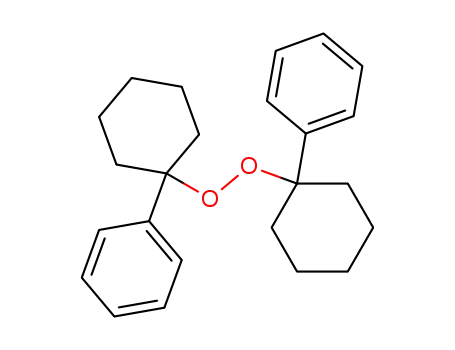
bis-(1-phenyl-cyclohexyl)-peroxide
-
109-65-9
1-bromo-butane
-
4747-13-1
α-methoxystyrene
942-92-7 Downstream products
-
63002-06-2
2-hydroxy-2-phenyl-heptanoic acid amide
-
4436-90-2
2-phenylheptane-2-ol
-
101888-02-2
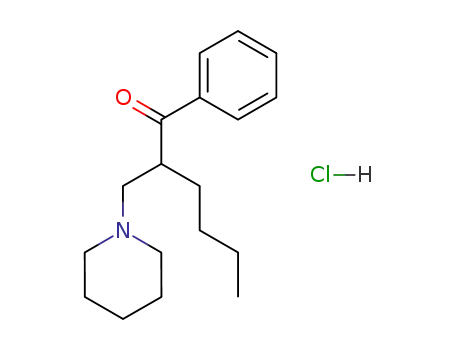
1-phenyl-2-piperidinomethyl-hexan-1-one; hydrochloride
-
101708-95-6
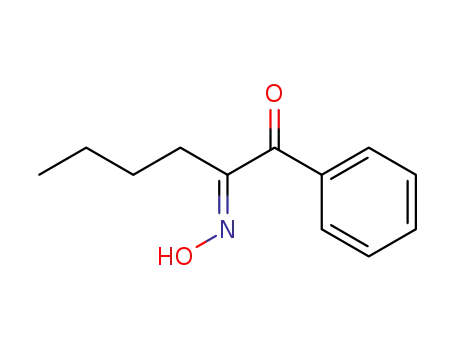
(E)-1-phenyl-1,2-hexanedione 2-oxime








