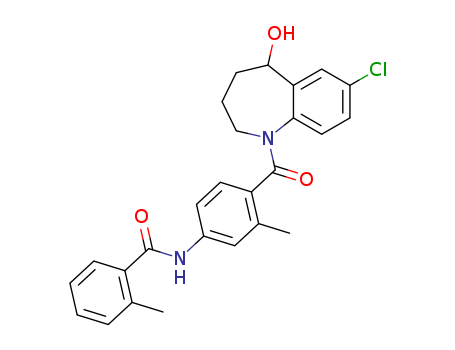
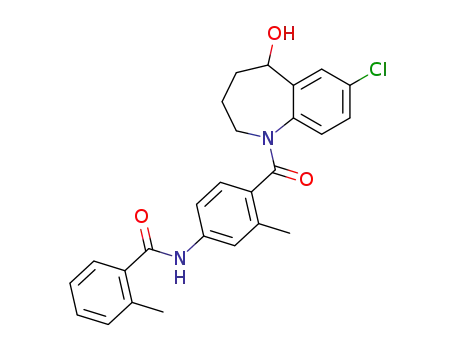
Tolvaptan
- CasNo:150683-30-0
- Molecular Formula:C<sub>26</sub>H<sub>25</sub>ClN<sub>2</sub>O<sub>3</sub>
- Purity:
- Molecular Weight:448.949
Product Details
Chinese Manufacturer Supply Tolvaptan 150683-30-0 On Stock with Competitive Price
- Molecular Formula:C26H25ClN2O3
- Molecular Weight:448.949
- Vapor Pressure:5.64E-15mmHg at 25°C
- Melting Point:219-222°C
- Refractive Index:1.663
- Boiling Point:594.4 °C at 760 mmHg
- PKA:13.00±0.70(Predicted)
- Flash Point:313.3 °C
- PSA:69.64000
- Density:1.311 g/cm3
- LogP:5.82110
Tolvaptan(Cas 150683-30-0) Usage
|
Synthesis |
Fugure 1: Synthesis of Tolvaptan |
|
Precautions |
Tolvaptan(SAMSCA) should be initiated and re-initiated in patients only in a hospital where serum sodium can be monitored closely. Too rapid correction of hyponatremia (e.g., >12 mEq/L/24 hours) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, affective changes, spastic quadriparesis, seizures, coma and death. In susceptible patients, including those with severe malnutrition, alcoholism or advanced liver disease, slower rates of correction may be advisable. Tolvaptan(SAMSCA) is contraindicated in the following conditions: Urgent need to raise serum sodium acutely Inability of the patient to sense or appropriately respond to thirst Hypovolemic hyponatremia Concomitant use of strong CYP 3A inhibitors Anuric patients Hypersensitivity (e.g. anaphylactic shock, rash generalized) to tolvaptan or its components |
|
Biochem/physiol Actions |
Tolvaptan (OPC 41061) is a potent, orally active non-peptide vasopressin V2 selective antagonist. IC50 = 3 nM at the rat V2 receptor; 29 times more selective for the V2 than for V1a. Tolvaptan has also been shown to inhibit the development of polycystic kidney disease in several animal models. |
|
Side effects |
The most common adverse events were thirst, dry mouth, asthenia, constipation, pollakiuria or polyuria, and hyperglycemia. The recommended starting dose is 15 mg daily with a daily 15-mg adjustment to a maximum of 60 mg daily to raise serum sodium concentration. Initiation should be in a hospital setting where serum sodium and volume status may be monitored since too rapid correction of hyponatremia (>12 mEq/L/24 h) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, spastic quadriparesis, seizures, coma, and death. In addition to avoiding concomitant use of strong CYP3A4 inhibitors, tolvaptan is contraindicated in settings of urgent need to raise serum sodium acutely, in patients with an inability to sense or appropriately respond to thirst, in hypovolemic hyponatremia conditions, and in anuric patients. |
|
references |
[1] miyazaki t, fujiki h, yamamura y, et al. tolvaptan, an orally active vasopressin v2-receptor antagonist-pharmacology and |
|
Brand name |
Samsca |
InChI:InChI=1/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31)/t24-/m1/s1
150683-30-0 Relevant articles
INTERMEDIATES AND METHODS FOR THE PREPARATION OF TOLVAPTAN AND ITS DERIVATIVES
-
, (2021/12/31)
The present invention relates to new int...
Preparation method of high-purity tolvaptan
-
, (2021/06/23)
The invention provides a preparation met...
MANUFACTURING METHOD OF TOLVAPTAN, SALT THEREOF AND SOLVATE
-
Paragraph 0093; 0095-0099; 0105-0155, (2020/04/18)
PROBLEM TO BE SOLVED: To provide a manuf...
Preparation method of high-purity tolvaptan
-
Paragraph 0023; 0024; 0025; 0026; 0027; 0028, (2018/03/01)
The invention discloses a preparation me...
150683-30-0 Process route
-
![4-bromo-2-methylphenyl-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]azepin-1-yl)methanone](/upload/2025/5/20998bd7-6cc0-4d64-aa72-267e579c28ed.png)
-
4-bromo-2-methylphenyl-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-benzo[b]azepin-1-yl)methanone

-
-
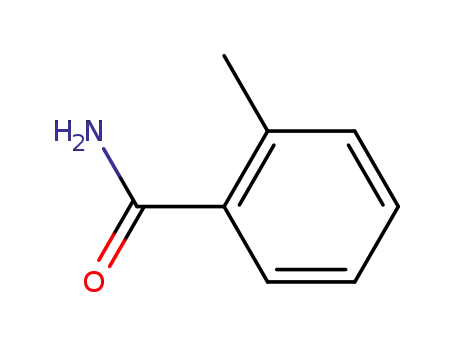
527-85-5
o-Methylbenzamid

-
-
150683-30-0
tolvaptan
| Conditions | Yield |
|---|---|
|
With
potassium phosphate;
In
toluene;
at 50 ℃;
for 20h;
Inert atmosphere;
|
81% |
-
![(±)-7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-ol](/upload/2025/5/f62a9d0c-286e-4f19-9461-8ae5d7a058a3.png)
-
1310357-40-4
(±)-7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-ol

-
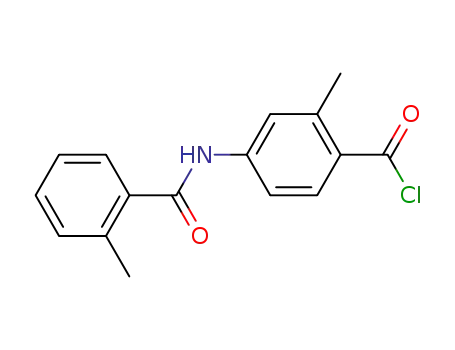
-
331947-69-4
2-methyl-4-(2-methylbenzoylamino)benzoic acid chloride

-

-
150683-30-0
tolvaptan
| Conditions | Yield |
|---|---|
|
With
2,6-dimethylpyridine;
In
N,N-dimethyl acetamide; water;
at 0 ℃;
for 2h;
Reagent/catalyst;
|
72% |
150683-30-0 Upstream products
-
137973-76-3
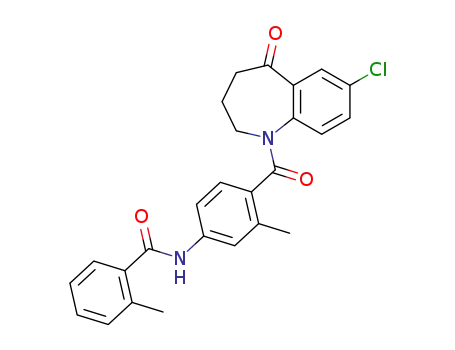
MOP-21826
-
804498-94-0
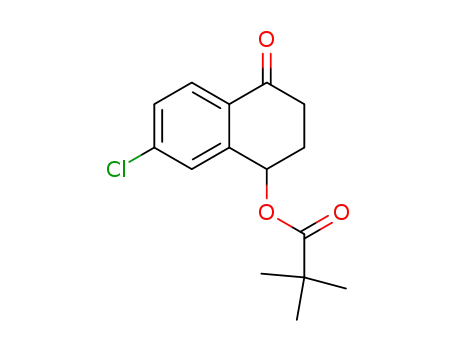
(rac)-7-chloro-4-oxo-1,2,3,4-tetrahydronaphthalen-1-yl pivalate
-
863762-10-1
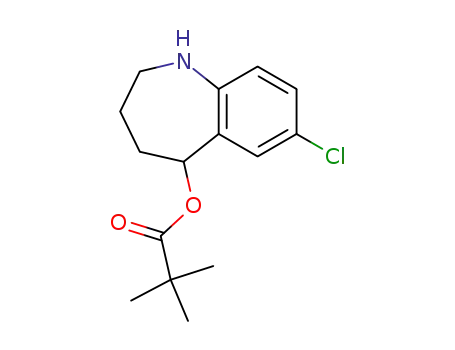
2,2-dimethylpropionic acid 7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl ester
-
863762-02-1
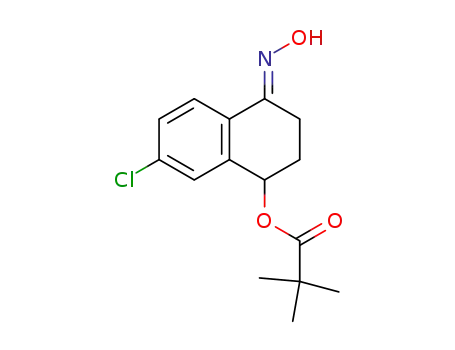
2,2-dimethylpropionic acid 4-[(E)-hydroxyimino]-7-chloro-1,2,3,4-tetrahydronaphthalen-1-yl ester
150683-30-0 Downstream products
-
942619-74-1
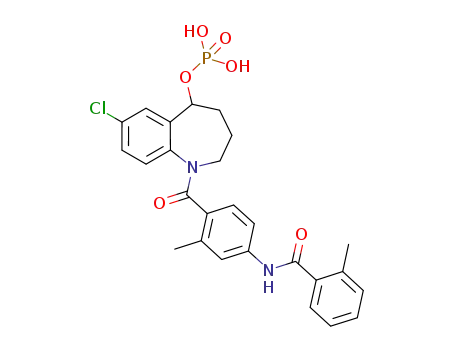
C26H26ClN2O6P
-
1056613-08-1
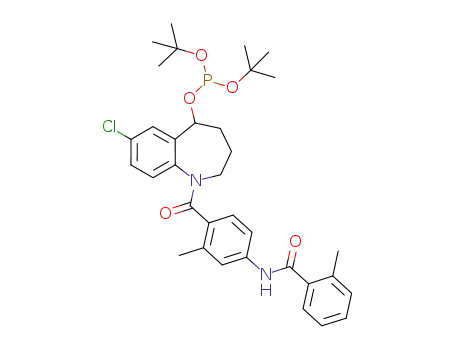
C34H42ClN2O5P
-
1056613-14-9
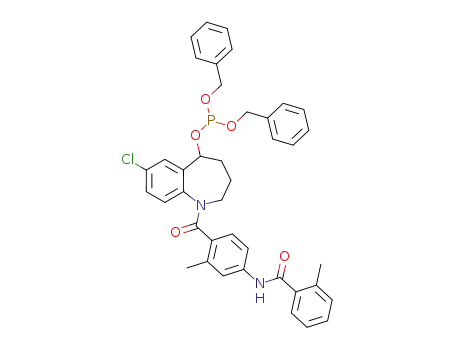
C40H38ClN2O5P
-
1094837-24-7
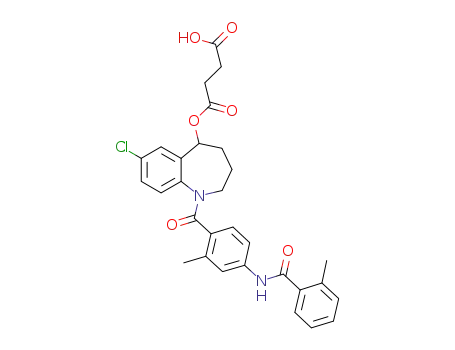
{7-chloro-1-[2-methyl-4-(2-methyl-benzoylamino)-benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl} succinate








