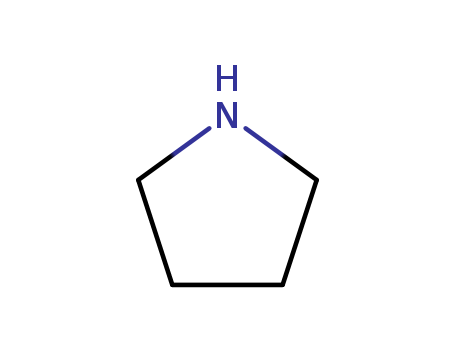
Pyrrolidine
- CasNo:123-75-1
- Molecular Formula:C<sub>4</sub>H<sub>9</sub>N
- Purity:
- Molecular Weight:71.1222
Product Details
Perfect Factory Offer Excellent quality Pyrrolidine 123-75-1 with Safe Shipping
- Molecular Formula:C4H9N
- Molecular Weight:71.1222
- Appearance/Colour:Colourless to pale yellow liquid
- Vapor Pressure:128 mm Hg ( 39 °C)
- Melting Point:-63 °C, 210 K, -81 °F
- Refractive Index:n20/D 1.443(lit.)
- Boiling Point:89.451 °C at 760 mmHg
- PKA:11.27(at 25℃)
- Flash Point:2.778 °C
- PSA:12.03000
- Density:0.866 g/cm3
- LogP:0.69860
Tetrahydro pyrrole(Cas 123-75-1) Usage
|
Preparation |
Pyrrolidine is formed by reduction of pyrrole. Via overall 5-endo-trig cyclizations of homoallylic tosylamides. Pyrrolidine can be produced from butanediol and ammonia, e.g., over an aluminum thorium oxide catalyst at 300°C or over a nickel catalyst at 200°C and 20 MPa under hydrogenation conditions. It can also be produced from THF and ammonia over aluminum oxide at 275-375°C. |
|
Air & Water Reactions |
Highly flammable. Very soluble in water. |
|
Reactivity Profile |
Tetrahydro pyrrole neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. An explosion occurred when a mixture of Tetrahydro pyrrole, benzaldehyde, and propionic acid was heated in an attempt to form porphyrins. |
|
Hazard |
Flammable, dangerous fire risk. Toxic by ingestion and inhalation. |
|
Health Hazard |
The acute toxicity of pyrrolidine is moderateon test animals. It is somewhat less toxicthan pyrrole. The vapors are an irritant tothe eyes and respiratory tract. The liquid iscorrosive to the skin. Contact with the eyescan cause damage. The oral LD50 value inrats is 300 mg/kg, while the inhalation LC50value in mice is 1300 mg/m3/2 h (NIOSH1986). |
|
Fire Hazard |
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Flammability and Explosibility |
Highlyflammable |
|
Safety Profile |
Poison by ingestion and intravenous routes. Moderately toxic by inhalation. Dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits hghly toxic fumes of NOx. |
|
Purification Methods |
Dry pyrrolidine with BaO or sodium, then fractionally distil it, under N2, through a Todd column (p 11) packed with glass helices. [Beilstein 20 H 159, 20 I 36, 20 II 79, 20 III/IV 2072, 20/1 V 162.] |
|
General Description |
Pyrrolidine (Tetrahydro pyrrole) is a versatile heterocyclic amine widely used in organic synthesis, acting as a catalyst in asymmetric Michael additions, facilitating nitrone formation through iminium activation, and serving as a nucleophile in amidinoethylation and SNAr reactions. It is also employed in the microwave-assisted synthesis of antimicrobial pyrrolidine derivatives and plays a key role in regioselective imidazole formation and amphiphilic allylation reactions. Its recyclability, stereoselectivity, and cooperative effects with other reagents make it valuable in green chemistry and pharmaceutical applications. |
|
Physical properties |
Pyrrolidine has a penetrating amine-type odor, reminiscent of ammonia and piperidine. It is easy to turn yellow when exposed to light or humid air, easily soluble in water and ethanol. It is nauseating and diffusive. |
|
Application |
Pyrrolidine is a heterocyclic compound used as a building block in the synthesis of wide range of pharmaceutical compounds, namely matrix metalloprotein inhibitors (MMPIs) and aminopeptidase N inhibitors (APNIs). It has been used for the synthesis of N-benzoyl pyrrolidine from benzaldehyde via oxidative amination. It may be used as a catalyst for the synthesis of N-sulfinyl aldimines from carbonyl compounds and sulfonamides.Pyrrolidine can also be used to synthesize:Taddol-pyrrolidine phosphoramidite, a ligand for rhodium-catalyzed [2+2+2] cycloaddition of pentenyl isocyanate and 4- ethynylanisole.H,4 PyrrolidineQuin-BAM (′PBAM′), a selective catalyst for the aza-Henry addition of nitroalkanes to aryl aldimines.1,2,3,3a,4,9-Hexahydropyrrolo[2,1-b]quinazoline by reacting with o-aminobenzaldehyde. |
|
Definition |
ChEBI: Pyrrolidine is a cyclic amine whose five-membered ring contains four carbon atoms and one nitrogen atom; the parent compound of the pyrrolidine family. It is a saturated organic heteromonocyclic parent, a member of pyrrolidines and an azacycloalkane. It is a conjugate base of a pyrrolidinium ion. |
|
Aroma threshold values |
Detection: 20.2 ppm |
|
Taste threshold values |
Taste characteristics at 50 ppm: ammonia and fishy, amine-like with seaweed and shellfish nuances. |
InChI:InChI=1/C4H9N/c1-2-4-5-3-1/h5H,1-4H2/p+1
123-75-1 Relevant articles
Photo-oxidation of L-Tyrosine, an Efficient 1,4-Chirality Transfer Reaction
Endo, Katsuya,Seya, Kazuhiko,Hikino, Hiroshi
, p. 934 - 935 (1988)
Dye-sensitized oxidation of L-tyrosine w...
-
Craig,Hixon
, p. 804,806 (1930)
-
-
Sakurai
, p. 374 (1936)
-
SALT EFFECTS ON THE KINETICS OF SUBSTITUTION OF THE PENTACYANO(PYRROLIDINE)FERRATE(II) ION
Pedrosa, Graciela C.,Hernandez, Norma L.,Katz, Nestor E.,Katz, Miguel
, p. 2297 - 2299 (1980)
Rate constants at 298.2 K for the releas...
Mechanistic Investigations of the Catalytic Formation of Lactams from Amines and Water with Liberation of H2
Gellrich, Urs,Khusnutdinova, Julia R.,Leitus, Gregory M.,Milstein, David
, p. 4851 - 4859 (2015)
The mechanism of the unique lactam forma...
Ion Confinement in the Collision Cell of a Multiquadrupole Mass Spectrometer: Access to Chemical Equilibrium and Determination of Kinetic and Thermodynamic Parameters of an Ion-Molecule Reaction
Beaugrand, Claude,Jaouen, Daniel,Mestdagh, Helene,Rolando, Christian
, p. 1447 - 1453 (1989)
Ions can be confined in an rf-only colli...
PHOTOSENSITIZED SINGLE ELECTRON TRANSFER INITIATED N-DEBENZYLATION. A CONVENIENT AND MILD APPROACH
Pandey, G.,Rani, K. Sudha
, p. 4157 - 4158 (1988)
A mild method of N-debenzylation via pho...
Novel β-galactosidase-specific O2-glycosylated diazeniumdiolate probes
Bedell, Barry,Bohle,Chua, Zhijie,Czerniewski, Alexander,Evans, Alan,Mzengeza, Shadreck
, p. 969 - 980 (2010)
Three β-galactosidase-specific nitric-ox...
N-trifluoroacetylamino alcohols as phosphodiester protecting groups in the synthesis of oligodeoxyribonucleotides
Wilk, Andrzej,Srinivasachar, Kasturi,Beaucage, Serge L.
, p. 6712 - 6713 (1997)
-
-
Takayama
, p. 138,139 (1936)
-
-
Brown,van Gulick
, p. 1046 (1956)
-
Surface ligands enhance the catalytic activity of supported Au nanoparticles for the aerobic α-oxidation of amines to amides
Chatterjee, Puranjan,Kanbur, Uddhav,Manzano, J. Sebastián,Sadow, Aaron D.,Slowing, Igor I.,Wang, Hsin
, p. 1922 - 1933 (2022/04/07)
The catalytic aerobic α-oxidation of ami...
A Lewis Base Nucleofugality Parameter, NFB, and Its Application in an Analysis of MIDA-Boronate Hydrolysis Kinetics
García-Domínguez, Andrés,Gonzalez, Jorge A.,Leach, Andrew G.,Lloyd-Jones, Guy C.,Nichol, Gary S.,Taylor, Nicholas P.
supporting information, (2022/01/04)
The kinetics of quinuclidine displacemen...
Highly economical and direct amination of sp3carbon using low-cost nickel pincer catalyst
Brandt, Andrew,Rangumagar, Ambar B.,Szwedo, Peter,Wayland, Hunter A.,Parnell, Charlette M.,Munshi, Pradip,Ghosh, Anindya
, p. 1862 - 1874 (2021/01/20)
Developing more efficient routes to achi...
Method for protecting sulfonyl of deamination amine
-
Paragraph 0054-0056, (2021/11/03)
The invention discloses a method for rem...
123-75-1 Process route
-
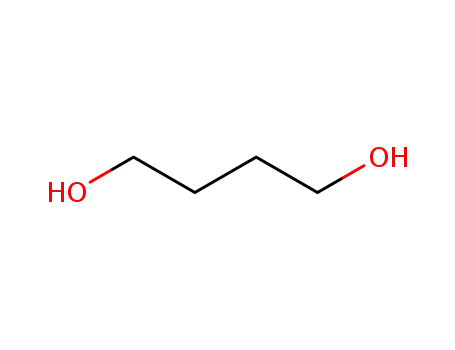
-
110-63-4
Butane-1,4-diol

-
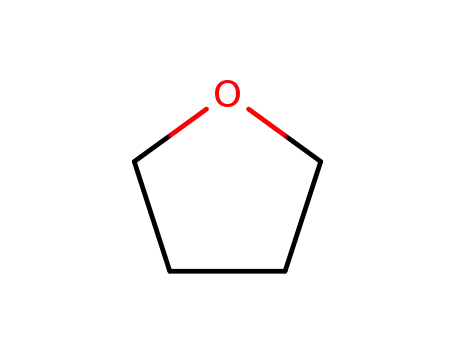
-
109-99-9,24979-97-3,77392-70-2
tetrahydrofuran

-
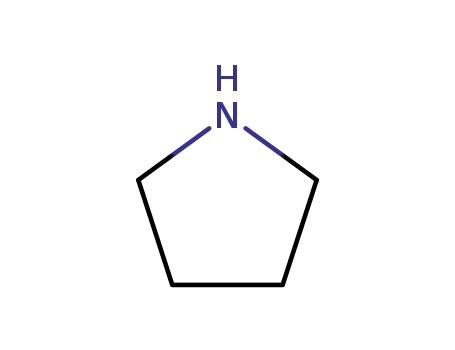
-
123-75-1
pyrrolidine

-
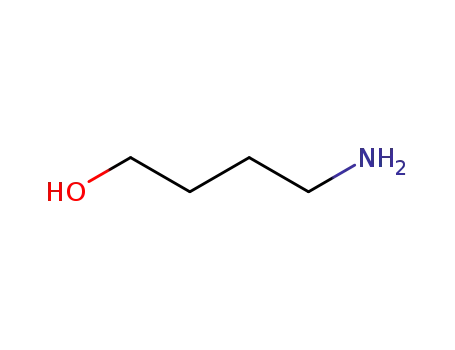
-
13325-10-5
4-Aminobutanol
| Conditions | Yield |
|---|---|
|
With
ammonia;
CrZMS-5;
at 300 ℃;
for 4h;
|
48.0 % Chromat. |
-
-
3686-43-9
3,6-dihydro-2H-[1,2]oxazine

-
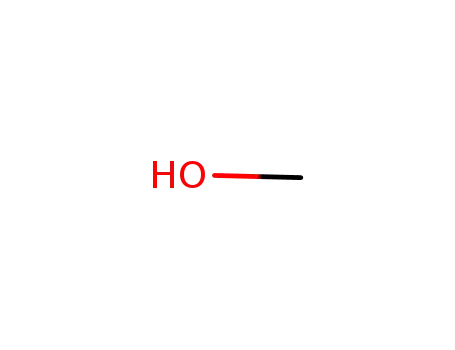
-
67-56-1
methanol

-

-
123-75-1
pyrrolidine

-
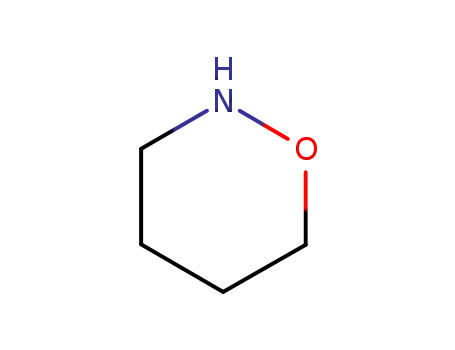
-
36652-42-3
1,2-oxazinane

-

-
13325-10-5
4-Aminobutanol
| Conditions | Yield |
|---|---|
|
Hydrogenation;
|
123-75-1 Upstream products
-
109-99-9

tetrahydrofuran
-
109-96-6
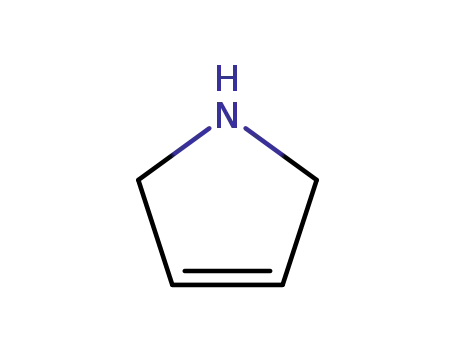
3-Pyrroline
-
5264-35-7
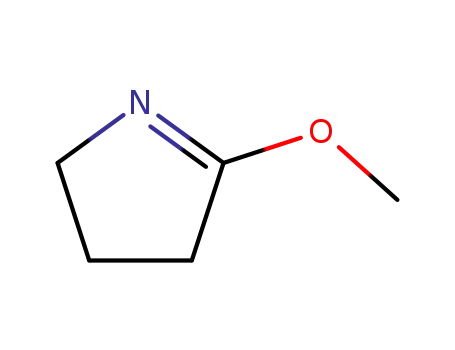
2-methoxy-1-pyrroline
-
50908-70-8
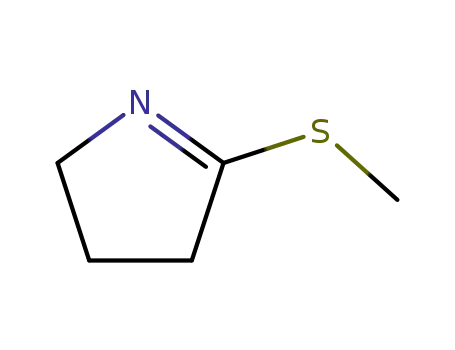
4-methylsulfanyl-3,4-dihydro-2H-pyrrole
123-75-1 Downstream products
-
2955-88-6
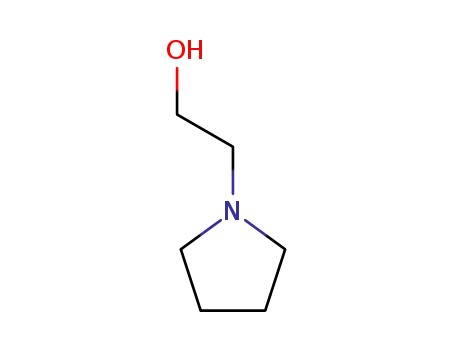
1-pyrrolidineethanol
-
3445-11-2
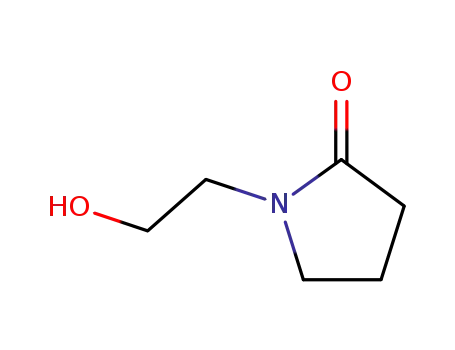
1-(2-hydroxyethyl)-2-pyrrolidinone
-
42302-16-9
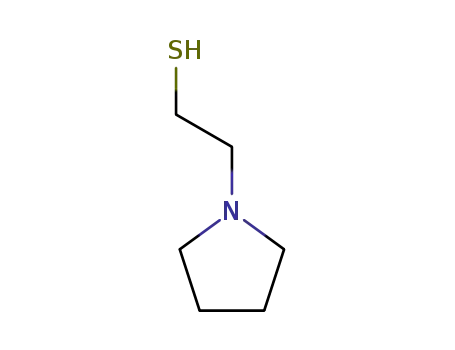
2-(pyrrolidin-1-yl)ethanethiol
-
10295-95-1
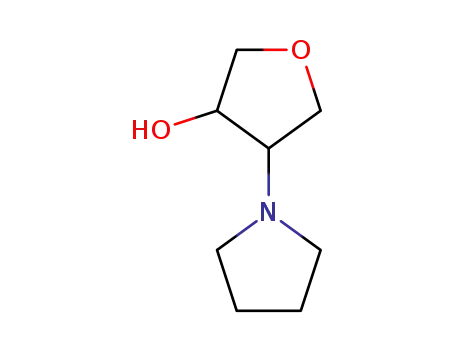
4-Pyrrolidino-tetrahydro-furan-3-ol








