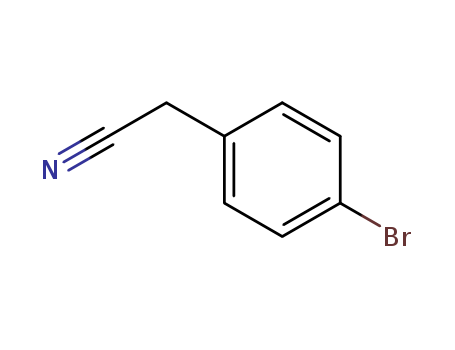
16532-79-9
- CasNo:16532-79-9
- Molecular Formula:C<sub>8</sub>H<sub>6</sub>BrN
- Purity:
- Molecular Weight:196.046
Product Details
Chinese factory supply 16532-79-9 16532-79-9 in stock with high standard
- Molecular Formula:C8H6BrN
- Molecular Weight:196.046
- Appearance/Colour:colorless to pale brown crystalline mass
- Melting Point:47-49 °C(lit.)
- Refractive Index:1.6550 (estimate)
- Boiling Point:286.2 °C at 760 mmHg
- Flash Point:126.9 °C
- PSA:23.79000
- Density:1.487 g/cm3
- LogP:2.51518
4-Bromophenylacetonitrile(Cas 16532-79-9) Usage
|
Safety Profile |
Poison by intravenous route. See also BROMIDES and NITRILES. When heated to decomposition it emits very toxic fumes of Br-, NOx, and CN-. |
|
General Description |
4-Bromophenylacetonitrile undergoes reduction using 1,1,3,3-tetramethyldisiloxane activated by a non-toxic titanium(IV) isopropoxide to yield 4-bromophenylethylamine. |
InChI:InChI=1/C8H6BrN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5H2
16532-79-9 Relevant articles
-
Griffin,R.W. et al.
, p. 2109 - 2116 (1964)
-
Rapid and Simple Access to α-(Hetero)arylacetonitriles from Gem-Difluoroalkenes
Hu, Dandan,Liu, Jiayue,Ren, Hongjun,Song, Jinyu,Zhang, Jun-Qi,Zhu, Guorong
supporting information, p. 786 - 790 (2022/01/28)
A scalable cyanation of gem-difluoroalke...
Radical trifunctionalization of hexenenitrile via remote cyano migration
Chang, Chenyang,Wu, Xinxin,Zhang, Huihui,Zhu, Chen
supporting information, p. 1005 - 1008 (2022/02/01)
A novel radical-mediated trifunctionaliz...
From Stoichiometric Reagents to Catalytic Partners: Selenonium Salts as Alkylating Agents for Nucleophilic Displacement Reactions in Water
Martins, Nayara Silva,ángel, Alix Y. Bastidas,Anghinoni, Jo?o M.,Lenard?o, Eder J.,Barcellos, Thiago,Alberto, Eduardo E.
supporting information, p. 87 - 93 (2021/11/03)
The ability of chalcogenium salts to tra...
Direct C(sp3)-H Cyanation Enabled by a Highly Active Decatungstate Photocatalyst
Kim, Kunsoon,Lee, Seulchan,Hong, Soon Hyeok
supporting information, p. 5501 - 5505 (2021/07/26)
A highly efficient, direct C(sp3)-H cyan...
16532-79-9 Process route
-
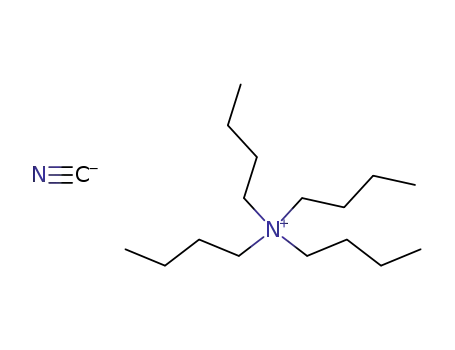
-
10442-39-4
tetra-n-butylammonium cyanide

-
![1-bromo-4-[(methoxymethyloxy)methyl]benzene](/upload/2025/5/43d34587-5116-44a9-8cb6-7c6e558e1f59.png)
-
130534-91-7
1-bromo-4-[(methoxymethyloxy)methyl]benzene

-
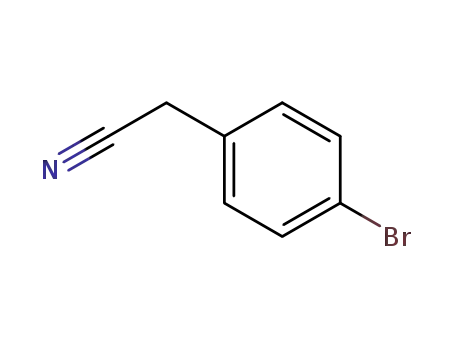
-
16532-79-9
4-Bromophenylacetonitrile
| Conditions | Yield |
|---|---|
|
With
phosphotungstic acid;
at 130 - 142 ℃;
for 0.0416667h;
chemoselective reaction;
Microwave irradiation;
Ionic liquid;
|
92% |
|
With
1-methyl-3H-imidazolium nitrate;
at 135 - 140 ℃;
for 0.05h;
Microwave irradiation;
|
88% |
|
With
1-(n-butyl)-3-methylimidazolium tetrachloroindate;
at 135 - 140 ℃;
for 0.0833333h;
chemoselective reaction;
Microwave irradiation;
Neat (no solvent);
|
84% |
-
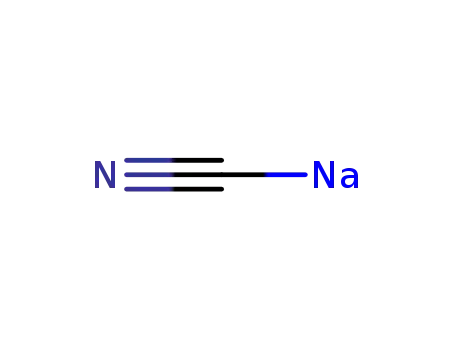
-
143-33-9,25596-52-5
sodium cyanide

-
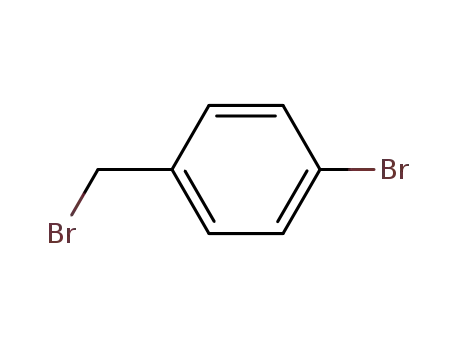
-
589-15-1
1-bromomethyl-4-bromobenzene

-
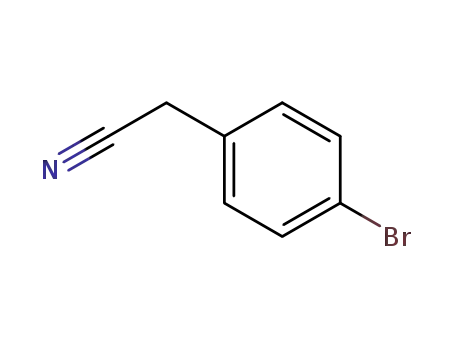
-
16532-79-9
4-Bromophenylacetonitrile
| Conditions | Yield |
|---|---|
|
In
water; N,N-dimethyl-formamide;
at 40 ℃;
for 12h;
|
89% |
|
In
ethanol; water;
for 4h;
Heating;
|
16532-79-9 Upstream products
-
99-99-0
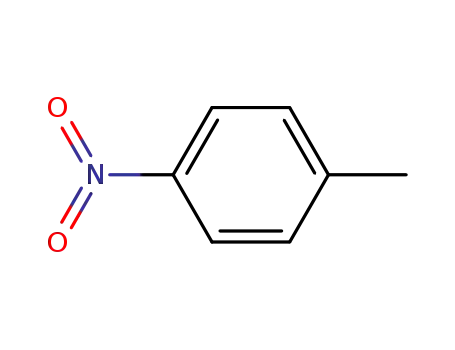
1-methyl-4-nitrobenzene
-
100246-20-6
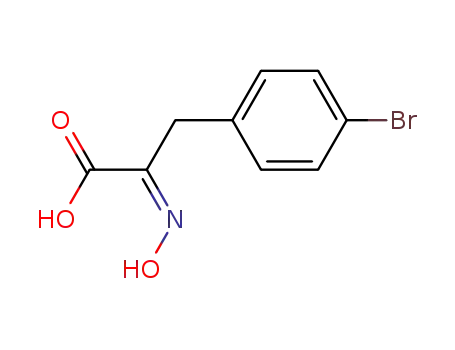
3-(4-bromo-phenyl)-2-hydroxyimino-propionic acid
-
108-24-7
acetic anhydride
-
106-49-0
p-toluidine
16532-79-9 Downstream products
-
96463-58-0
2-(4-bromo-phenyl)-3c-(5-bromo-[2]thienyl)-acrylonitrile
-
102661-91-6
4-(4-bromo-benzyl)-quinoline
-
107413-80-9
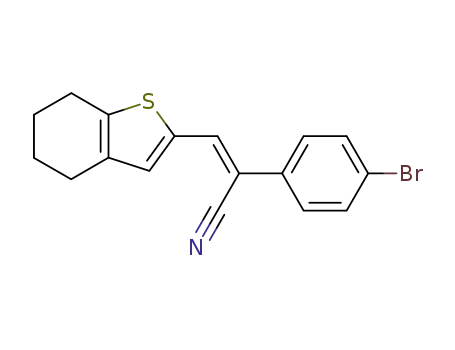
2-(4-bromo-phenyl)-3c-(4,5,6,7-tetrahydro-benzo[b]thiophen-2-yl)-acrylonitrile
-
14062-25-0
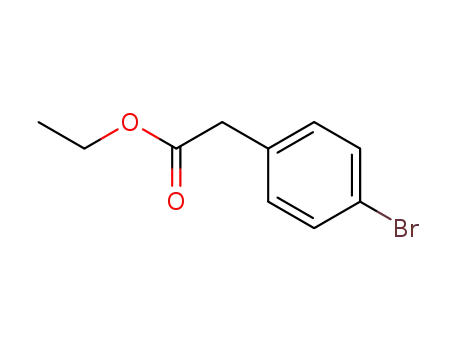
Ethyl 4-bromophenylacetate








